Find all the information you need
| Units of measurement (mm) |
MAX
|
MIN
|
|---|---|---|
| WIDTH | 1600 | 250 |
| LENGTH | 8000 | 300 |
| THICKNESS | 4,0 | 0,3 |
| WEIGHT(kg) | 18000 |
| Units of measurement (mm) |
MAX
|
MIN
|
|---|---|---|
| WIDTH | 1560 | 500 |
| THICKNESS | 20,0 | 0,5 |
| No OF FINISH | We may deliver the finishing our customers desire | |
| Units of measurement (mm) |
MAX
|
MIN
|
|---|---|---|
| THICKNESS | 15 | 1,0 |
| LENGHT | 6000 | 200 |
| WIDTH | 200 | 15 |
| Units of measurement (mm) |
MAX
|
MIN
|
|---|---|---|
| THICKNESS | 2 | 0,4 |
| INSIDE CIRCLE DIAMETER | 500 | 270 |
| INSIDE DIAMETER OF SPLIT | 10 |
| Units of measurement (mm) |
MAX
|
MIN
|
|---|---|---|
| WIDTH | 2100 | |
| LENGTH | 6500 | |
| THICKNESS | 30 | 1 |
| Units of measurement (mm) |
MAX
|
MIN
|
|---|---|---|
| LENGTH | 3000 | 100 |
| THICKNESS | 6 | 0,4 |

| Units of measurement (mm) |
MAX
|
MIN
|
|---|---|---|
| WIDTH | 1600 | 250 |
| LENGTH | 8000 | 300 |
| THICKNESS | 4,0 | 0,3 |
| WEIGHT(kg) | 18000 |
| Units of measurement (mm) |
MAX
|
MIN
|
|---|---|---|
| WIDTH | 1560 | 500 |
| THICKNESS | 20,0 | 0,5 |
| No OF FINISH | We may deliver the finishing our customers desire | |
| Units of measurement (mm) |
MAX
|
MIN
|
|---|---|---|
| THICKNESS | 15 | 1,0 |
| LENGHT | 6000 | 200 |
| WIDTH | 200 | 15 |
| Units of measurement (mm) |
MAX
|
MIN
|
|---|---|---|
| THICKNESS | 2 | 0,4 |
| INSIDE CIRCLE DIAMETER | 500 | 270 |
| INSIDE DIAMETER OF SPLIT | 10 |
| Units of measurement (mm) |
MAX
|
MIN
|
|---|---|---|
| WIDTH | 2100 | |
| LENGTH | 6500 | |
| THICKNESS | 30 | 1 |
| Units of measurement (mm) |
MAX
|
MIN
|
|---|---|---|
| LENGTH | 3000 | 100 |
| THICKNESS | 6 | 0,4 |

Steels are the iron alloys with 2% maximum carbon content that constitute over the 80% of the industrial alloysAlloy is the material that is composed from various chemical elements. When it is solid, it is characterized by the participation of all the elements into the crystal structure. That is, the material is crystalline and in this crystal the atoms of its constituents are arranged in space as if they are atoms of the same kind. The crystal structure reflects the geometrical construction of a chemical element. It is called “crystal” in the case of a solid material that exhibits regular geometrical arrangement of its structural constituents. Depending on the geometrical arrangement the atoms or other particles of the element form, the crystal structure is distinguished into seven lattice systems: triclinic, monoclinic, orthorhombic, rhombohedral, tetragonal, hexagonal and cubic.. The latter is attributable to their low cost of production and the relative ease of producing steel in large quantities with accurate standards - specifications. The following three factors play a determinant role in the widespread use of steel.
(a) the high global reserves of mineralMineral is a naturally occurring solid chemical substance formed through biogeochemical processes, having characteristic chemical composition, highly ordered atomic structure (usually crystalline), and specific physical properties. By comparison, a rock is an aggregate of mineral and/or mineraloids and does not have a specific chemical composition. (the earth crust consists of around 4% iron FeIron the chemical element symbolized by Fe is a metal with atomic number 26, melting and boiling temperatures 1535°C and 2750°C respectively. It is the forth most abundant element on the earth’s crust after oxygen (O), silicon (Si), and aluminum (Al). Moreover, it is the metal with the widest use, especially in the form of its two most important alloys, that of steel and that of cast-iron (iron alloy with carbon °C > 2,1 %).) which can be easily converted into the metallic condition, together with the availability coming from the recycling of scrap.
(b) the iron’s melting point (1539°C) allows the thermal activation of processes at temperatures (Τ.>400°C) which can be achieved relatively easily and be controlled by industrial manufacturing.
(c) the allotropy of iron and the transformation of steel’s phases (i.e. martencitic transformation) allow the formation of a great variety of microstructures which leads in turn to a respectively large range of mechanical properties. Elements that are characterized by the condition of allotropy are those that appear with more than one natural forms, due to the fact that their atoms are matched with one another in various ways (i.e. graphite and diamond constitute manifestation for the allotropy of carbon).
All the above-mentioned, make steel as the most important and popular material in mechanical engineering. Much of this stems from the allotropy of iron. The formation of the structure and the properties shown by different types of steel are achieved through industrial procedures named as heat treatments (most known being that of annealing). The greatest variety of steel microstructures appear during the transformation of austenite while it is being cooled. So, according to the allotropy of iron, we have the phase of α-Fe BCC (body centered cubic crystal lattice) dominating up to 910°C, the phase of γ- Fe FCC (face centered cubic crystal lattice) between 910 and 1400°C and α-Fe reappearing from 1400°C and to the melting point.
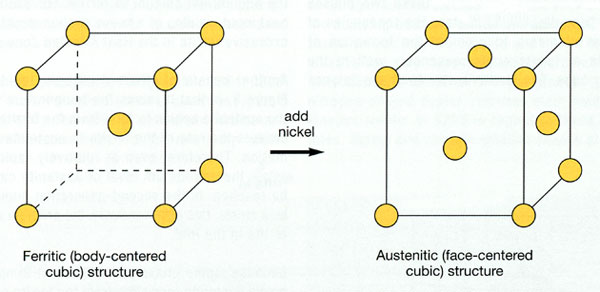
1. By adding nickel, the crystallographic structure changes from body-centered cubic (little or no nickel) to face-centered cubic (at least 6% nickel – 300 series).
The solid solution α-Fe with carbon is named ferrite, while the corresponding solid solution γ-Fe with carbon, austenite. Main difference between the two phases is the solubility of carbon which is much higher in the austenite than the ferrite. For example, the austenite can dissolve 2% carbon while the ferrite just 0,02% by weight.
Even though the most important alloying element in steel is carbon, in most cases several other alloying elements are added for various reasons. Thus, in most types of steel we will observe the presence of Mn and Si as well as that of Cr, Ni and Mo. The role of an alloying element is compound. It influences the solid solubility of the other alloying elements, the thermodynamic stability of the phases and in general, the formation of the steel’s microstructure together with its physical and mechanical properties. More specifically, the elements are distinguished into two categories according to their tendency to promote either the austenitic or the ferritic microstructure. The most important impacts of the alloying elements are mentioned below in brief.
* The present text has been registered at a notary office for the intellectual rights' protection.
Chromium (Cr) is a highly carbide forming element. Chromium carbides raise the steel’ s toughness and increase scaling and wear resistance. Moreover, chromium increases the resistance to oxidation (including that caused by high temperatures) and the resistance to corrosion. It constitutes the main alloying element for stainless steel.
Molybdenum (Μο) is a strong carbide former. It gives steel extra resistance to corrosion and particularly to pitting and crevice corrosion – these terms are explained later. The high melting point of molybdenum makes it important for giving strength to steel at high temperatures.
Titanium (Ti) and Niobium (Nb) are both elements that form carbides and raise steel’s strength and resistance – even at high temperatures. Both of them play the role of stabilizer and contribute in reducing the risk of intergranular corrosion – it is explained later.
Silicon (Si) and Aluminum (Al) are two elements used for the deoxidization of steel. High silicon content leads to lower ductility. Both of them increase resistance to oxidation in high temperature environments.
Nickel (Ni) in stainless steels has no direct influence on corrosion resistance. Thanks to nickel austenitic stainless steels exhibit a wide range of ideal mechanical properties, like excellent ductility and toughness, even at high strength levels. These properties are retained up to cryogenic temperatures.
Carbon (C) is an important alloying element in all-ferrous metal based materials. Carbon is a very strong austenitizer and increases the strength of steel. By increasing its content we raise hardness and toughness and make the steel heat treatable by quenching and tempering to develop the martensite phase. However, resistance to fracture, ductility and weldability are reduced with higher carbon content.
Nitrogen (N) raises the strength and the resistance of steel, while at the same time it reduces its ductility. More specifically, it improves the mechanical properties of austenitic and duplex stainless steels, increasing at the same time their resistance to localized corrosion like pitting or intergranular.
Manganese (Mn) is essential to steel making because of two key properties: its ability to combine with sulphur and its powerful deoxidation capacity. Manganese will combine preferentially with sulphur to form manganese sulphide (Mn S) which favour workability and weldability of steel. Moreover, its presence increases the hardenability of the steel. Its ability to stabilize the austenite in steel is used to substitute nickel in some austenitic stainless steels.
Copper (Cu) improves resistance to corrosion from sulphuric acid.
Neutral elements
Sulphur (S) and Phosphorous (P) are undesired impurities. They act on reducing ductility, weldability. At the same time they reduce resistance to corrosion and increase steel’s inclination to cracking.
Stainless steel is an alloy of iron, carbon and chromium with minimum content of chromium at least 10,5 % by weight. Chromium creates an adherent, insoluble film on the surface of the steel that shields the metal substrate from uniform and localized attack. This protective film is called passive layer or passive film and constitutes a very fine layer on the surface of the steel (its thickness is 5-15 nm). Apart from chromium, stainless steels may contain and other alloying elements like nickel Ni, molybdenum Mo, manganese Mn etc (we examined them earlier).
They are widely used into several applications that require among others resistance to corrosion, effective relation between cost and life cycle, for their aesthetic or hygienic properties. Stainless steels apart from their resistance to corrosion, they also enjoy higher mechanical properties in comparison with other common types of steel. Yet, they are harder and more difficult to work with. An additional characteristic is their lower thermal conductivity.
Stainless steels are distinguished according to the dominant phase in their crystal lattice.
Austenitic stainless steels They are stainless steels with main phase that of the austenite (γ-Fe). Austenite is the allotropic form of iron, which crystallizes according to the face centered cubic system (FCC). Austenitic stainless steels contain very low quantity of carbon (usually <0,08% C, but some contain up to 0,15% C) and at least 16% Cr. The austenite is stabilized with the addition of Ni or and Mn, and remains into stable phase in all the temperature breadth from the alloy’s melting point until well below 0℃. Due to the austenite’s non-magnetic nature, austenitic stainless steels are not magnetic.
The most common austenitic stainless steels are 18/8 (18% Cr, 8% Ni) and 18/10 (18% Cr, 10% Ni), which belong in 300 series, according to the American standards AISI-SAE. The steels AISI 316 display higher resistance to corrosion and are characterized by the presence of molybdenum (around 2%). The types 304L and 316L contain the lowest quantity of carbon (< 0,03%) resulting in better performance during welding. Generally, the types of the 300 series present good corrosion resistance, excellent forming capability, low yield strength Mechanical properties of a metallic alloy are those that describe the material’s ability to compress, stretch, bend, scratch, dent or break. The most commonly used criteria for evaluating mechanical characteristics are:
a. Strength: the degree of resistance of a material to deformation. Two critical values are generally considered:
yield strength, or the stress the material can be subjected to before permanent plastic deformation occurs
tensile strength, or the stress it can be subjected to before rupture/failure
15. UTS (ultimate tensile strength) is measured in MPa (1Mpa=1N/mm3=145PSI=0.1 kg/mm3) and represents maximum resistance at failure. YS (yield strength) refers to the beginning of the “plastic” phase, where elongation no longer disappears when the stress is removed.
b. Hardness: the degree of resistance to indentation by an applied load.
c. Toughness: the capacity to absorb deformation energy before fracture.
d. Ductility (or plasticity): the ability to deform plastically without fracturing.
16. Rm=ultimate tensile strength, Rp02=yield strength and A5/A80=elongation to fracture. , relatively high tensile strength Mechanical properties of a metallic alloy are those that describe the material’s ability to compress, stretch, bend, scratch, dent or break. The most commonly used criteria for evaluating mechanical characteristics are:
a. Strength: the degree of resistance of a material to deformation. Two critical values are generally considered:
yield strength, or the stress the material can be subjected to before permanent plastic deformation occurs
tensile strength, or the stress it can be subjected to before rupture/failure
15. UTS (ultimate tensile strength) is measured in MPa (1Mpa=1N/mm3=145PSI=0.1 kg/mm3) and represents maximum resistance at failure. YS (yield strength) refers to the beginning of the “plastic” phase, where elongation no longer disappears when the stress is removed.
b. Hardness: the degree of resistance to indentation by an applied load.
c. Toughness: the capacity to absorb deformation energy before fracture.
d. Ductility (or plasticity): the ability to deform plastically without fracturing.
16. Rm=ultimate tensile strength, Rp02=yield strength and A5/A80=elongation to fracture. and good weldability, providing a wide range of applications.
Apart from the well-known 300 series, there exist the less resistant types of the 200 series with manganese. These new types use different chemistry which is differentiated by the lower chromium content (<15%) and the much lower nickel content. The reduction of nickel with the simultaneous addition of manganese limits the quantity of chromium that can be added, affecting negatively therefore the resistance to corrosion. It has been mentioned earlier that the addition of nickel is the principal way for protecting the austenitic structure in stainless steel. However, the addition of manganese in combination with the presence of nitrogen may bring about the same results – albeit at lower cost. The chromium–manganese types are characterized by notably lower nickel content and by the existence of manganese and often that of nitrogen and copper (both of which share the capability of promoting the austenitic structure). The addition of nitrogen results to a better stabilization of the austenitic phase, allowing therefore the addition of more chromium. Nitrogen also acts as a hardening factor. We use to refer to the types of the 200 series simply by mentioning their nickel content – like 4% Ni and 1% Ni. The most popular types are 201 (1% Ni, min 15% Cr, max 0,1% C) and 202 (4% Ni, min 16% Cr, max 0,08% C).
Apart from the fact that the chromium–manganese stainless steels have a lower cost, at the same time they present good forming capability and good corrosion resistance depending on their chemistry. Their characteristic advantage is their higher mechanical properties compared to those of the classical 300 series (i.e. 304), something that allows the reduction of the gauge of the material used and thus the reduction of the weight. However, we draw your attention to the fact that the 200 series (especially types with high nitrogen content) are more difficult to form. The addition of copper is a solution to this problem, since it allows the reduction of nitrogen with the content of nickel and chromium remaining stable.
The rise in the popularity of the 200 series was related to the instability of the nickel value (especially during periods of sharp increases in its price), as well as to the progress in the technology used for the steel’s production. At the same time, constant pressure for the reduction of cost, especially in the Asian market, has led to the development of austenitic types with low nickel and chromium content which often do not satisfy any international standards. Actually, several chromium–manganese types are distinctive of specific mills’ production and are defined simply from a title given by each producer. Therefore, it is suggested to manufactures that examine their potential use to take the advice of credible suppliers who have the right information and are capable of supplying good quality products of reliable origin. Moreover, we note that the potential user has the possibility to choose alternative solutions among the 400 and 300 series.
Concluding the 200 series presentation, we mention below some applications were the experience has proven their positive performance (mainly for the types with 4% Ni). Such applications include cutlery and cooking utensils, home sinks, catering equipment, trucks’ structural parts, bushes’ chassis and constructions in the sugar industry, as well as architectural constructions that are not near coasts.
Finally, we make a reference to the super austenitic stainless steels with very high nickel content Ni (>20%) and molybdenum Μο (>6%), for strong resistance to the corrosion caused by acids, chlorine and chloride solutions. The most popular type of this category is AISI 904L (19-23% Cr, 23-28% Ni, 4-5% Mo).
These are stainless steels with main phase that of ferrite (α-Fe) or martensite (phase that results from the rapid cooling of the austenite). They contain 10,5-27% chromium, with very little or none at all nickel (<2%). Some of them have molybdenum or titanium and niobium.
The martensitic stainless steels contain 12-17% chromium and have higher carbon content. These kinds of stainless steels are subjected to specific heat treatment that leads to an increase of their hardness. They are used for the manufacturing of turbine propellers, cutlery, knife blades etc.
The ferritic stainless steels share the same advantages (mechanical properties and corrosion resistance) with the austenitic types. In some cases their performance is superior to that of the austenitics. In short, we mention below the special advantages of the ferritic stainless steels.
Ferritic grades fall into five groups – three families of standard grades and two of "special" grades.
Group 1 (409/410L) has the lowest chromium content (10-14%) of all stainless steels and is also the least expensive. This group can be ideal for non–or lightly–corrosive environments or applications where slight localized rust is acceptable. Typical applications are automotive exhaust–system silencers for the type 409, while 410L is often used for containers, buses, coaches and LCD monitor frames.
Group 2 (430) is the most widely used family of ferritic alloys. Having a higher chromium content (14-18%), group 2 grades show greater resistance to corrosion and behave most like austenitic grade 304. Typical uses include washing–machine drums, household utensils, dishwashers, pots and pans and indoor panels. Generally speaking, we note the fact that 430 is suitable to replace 304 in some applications where the use of the latter is judged as an excessive (over-alloyed) and expensive choice (i.e. catering equipment).
Group 3 includes 430Ti, 439, 441. Compared with group 2, these grades show better weldability and formability. Their behavior is even, in most cases, better than that of 304 austenitic grades. This group has chromium content between 14 and 18% and is characterized by the presence of stabilizing elements like titanium and niobium. Sinks, exchanger tubes, exhaust systems (longer life than with type 409) and catering equipment are some of the typical applications. Group 3 grades can replace type 304 in applications where this grade is an over – specification. 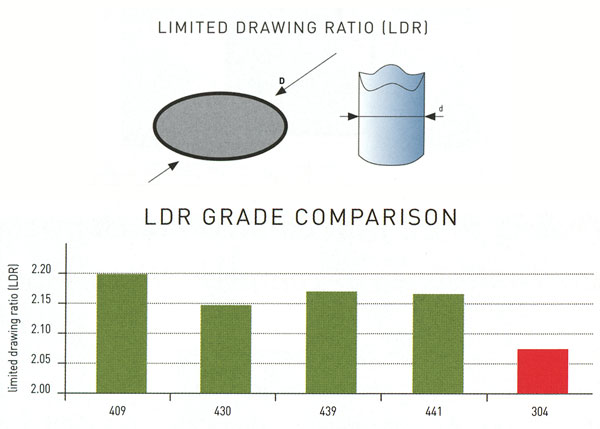
2. Τhe Limited Drawing Ratio (LDR) is an important deep-drawability parameter. It refers to the quotient of the maximum blank diameter (D) that can be deep drawn into a cylinder in one step and the diameter (d) of that cylinder. LDR=D/d.
Group 4 includes types 434, 436, 444, etc. These grades have added molybdenum, for extra corrosion resistance. The chromium content lies between 14 and 20%. Typical applications include hot water tanks, solar water heaters, visible parts of exhaust systems, electrical – kettle and microwave oven elements, automotive trim and outdoor panels, etc. Type 444’s corrosion resistance level can be similar to that of type 316.
Group 5 (types 446, 445/447 etc) has additional chromium (18-30%) and contains molybdenum, for extra corrosion and scaling (oxidation) resistance. The grades of this group are superior to type 316 in respect of these properties. Typical uses include applications in coastal and other highly corrosive environments.
Duplex (austenitic-ferritic) stainless steels are comprised by austenite and ferrite in proportions from 50:50 to 40:60. Usually, they contain 19-28% Cr, <5% Mo and a bit of Ni (1,5-7% depending on the grade). Thanks to their high content of chromium, nitrogen and molybdenum they show excellent corrosion resistance, enjoying at the same time the advantage of having higher mechanical properties in comparison to the other families.
For instance, the type S32101 has 90% higher mechanical properties than 304, allowing thus the safe reduction of the material’s thickness by 30% (on average).
On top of that, due to their low nickel content they have a more stable price than the austenitic stainless steels. They are distinguished into the following groups.
Metals, excluding precious metals such as gold or platinum, found in their natural state, are always extracted from minerals (oxides, sulphides, various chemical compounds). Most metals tend to deteriorate in contact with the atmosphere, water or other natural or industrial environments and gradually return to their original compound state. Metal corrosion is equivalent to a chemical dissolution or electrochemical dissolution of a metal or an alloy, causing material losses dependent on the type of material and the nature of the environment (chemical composition, temperature, etc). In the case of steels, the term corrosion is applied to phenomena developed in the liquid phase (aqueous or organic solutions, salts or molten metals), as opposed to oxidation (or "dry" corrosion) which occurs at high temperature.
Corrosion can be limited, even avoided, if the chemical reactions that take place do not lead to the formation of a soluble type of oxide, but rather to the appearance of a stable, protective oxide layer or compound. If these compounds form a thick enough compact layer (called a "passive film" or "passive layer") on the surface of the metal, the risk of corrosion is considerably reduced by the phenomenon of passivation.
Stainless steels are a modern solution to the problem of corrosion. The addition of highly oxidisable elements such as chromium increases the tendency to form stable oxides. Paradoxically, it can be stated that the corrosion resistance of stainless steels is due to the particularly oxidisable nature of one of its elements, chromium, provided that it is present in the proportion of at least 10,5%. Corrosion resistance is obtained by the spontaneous formation in the open air of a very thin passive film, no thicker than about ten nanometers (the equivalent of a protection given by a sheet of paper placed on a 20-storey building). The stability of the passive film is the factor determining the corrosion resistance of stainless steels and it depends on various factors, the main ones being: nature of the corrosive environment, composition of the steel, surface condition, any contact with other materials. When stainless steel is used correctly and the appropriate grade chosen to suit the surrounding medium, the passive film is spontaneously regenerated after accidental damage.
Wrong choices and the prevalence of various conditions, may lead to failure of stainless steels and the emergence of corrosion. In their case, corrosion may not appear with the obvious rust visible in common steels. The effects of corrosion often propagate with fast rate and destructive results. Different media can cause different types of corrosion attack that may vary in nature and appearance, and several forms of corrosion can occur on stainless steels. Below, follows a short presentation of the most common forms of corrosion that can attack stainless steels.
Uniform corrosion occurs when all, or at least a large section, of the passive layer is destroyed. Corrosion reactions then cause a more or less uniform removal of metal from the unprotected surface. Attack by uniform corrosion on stainless steels occurs mainly in acids or in hot alkaline solutions (i.e. sodium and potassium).
Stainless steels generally show good resistance in oxidizing acids such as nitric acid, but are not always able to maintain their passive layer in non-oxidizing acids (like hydrochloric and hydrofluoric acids). Substances, which are neither reducing nor oxidizing, may also affect the corrosivity of acid solutions. Most important are halides, such as chlorides and fluorides, the presence of which increases the corrosivity of both organic and inorganic acids. Even small amounts of halides may affect the corrosion resistance of stainless steels. The resistance to uniform corrosion is generally improved by higher chromium content, since chromium is essential for ensuring the passivity of stainless steels. Nickel is also important as it helps reduce the corrosion rate of depassivated steel. The presence of molybdenum and copper also has a positive effect and enhance the steel’s resistance.
In selecting material, it is important to consider all variations in temperature and chemical composition that might occur in the process environment. 
3. Uniform corrosion on the outside of a steam tube that has been exposed to sulphuric acid.
There are media in which the passive layer might break down locally, while the rest of the layer remains intact. Such media cause so-called localized corrosion. Pitting corrosion is one type of localized corrosion (later we describe more types) and is characterized by attack at small discrete areas. Attack of this kind occurs mainly in neutral or acidic solutions containing chloride.
Chloride ions facilitate a breakdown of the passive layer. A break in the passive film may be considered as a galvanic cell, in which the bare metal becomes the anode while the surrounding area, with an undamaged passive layer, becomes the cathode. This unfavourable anode-to-cathode surface area ratio causes rapid corrosion of the anode.
When the metal corrodes, dissolved metal ions generate an environment with a low pH and chloride ions migrate into the pit to balance the positive charge of the metal ions. Thus the environment inside a growing pit gradually becomes more aggressive and repassivation becomes less likely. As a result, pitting attack often propagates at a high rate, thereby causing corrosion failure in a short time. Since the attack is small at the surface and may be covered by corrosion products, a pit often remains undiscovered until it causes perforation and leakage. 
4. Pitting attack in the heat affected zone inside a but-welded stainless steel pipe.
Crevice corrosion occurs under the same conditions as pitting, i.e. in neutral and acid chloride solutions. However, attack starts more easily in a narrow crevice than on an unshielded surface. The chemical reactions occurring naturally on a stainless surface in an aqueous environment consume oxygen. In the stagnant solution inside a crevice, the supply of new oxidant is restricted. The composition of the solution within the crevice might thus gradually become different from that of the ambient solution. This difference in composition increases the risk for corrosion. Small amounts of dissolved metal ions cause a decrease of the solution pH inside the crevice and the presence of chlorides facilitates an activation of the metal surface. The bare metal surface behaves anodically to the passive areas and the attack propagates according to the same mechanisms as in the case of pitting. Crevice corrosion may be strong even at relatively low temperature.
High chloride concentrations and low pH increase the probability of pitting and crevice corrosion, as do high temperatures and stagnant solutions. The resistance of stainless steels to these types of corrosion increases with increasing contents of chromium and molybdenum. For austenitic and duplex grades, high nitrogen content is also very beneficial. We draw the attention to the fact that a smooth and clean surface generally exhibits the best corrosion resistance. Since crevices are the most vulnerable areas to corrosion, it may be advantageous to use more highly alloyed materials (i.e. 316 instead of 304) in joints (like flanges, bolts etc) than in the pipes of a piping system. The resistance of a construction to crevice corrosion may also be enhanced by overlay welding with a more corrosion resistant material at potential sites of crevice corrosion. Finally, we note that pitting attack initiates more easily in stagnant solutions than in flowing media and that is why equipment should be designed in a way that permits complete drainage.
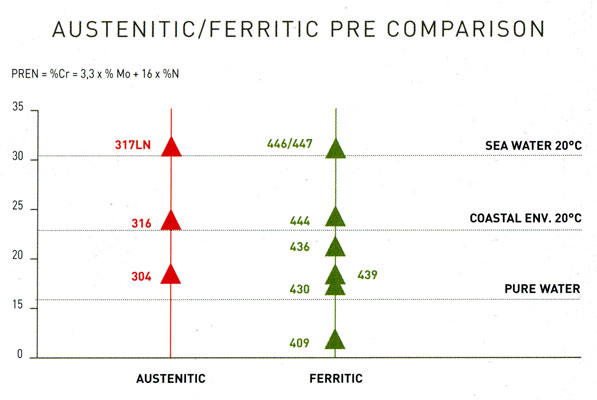
For the right examination and decision of which type to choose for avoiding the most common form of corrosion - which is pitting - we use the so-called PRE factor. PRE (Pitting Resistance Equivalent) constitutes a measurement of a stainless steel grade's relative resistance to pitting corrosion. The highest is a grade's PRE number, the highest corrosion resistance it enjoys. The formula that gives us the PRE of each grade is the following
PRE = % Cr + 3.3 % Mo + 16 % N
and shows that the resistance to corrosion is the result of the steel's chemical composition and not its atomic structure (austenitic or ferritic). Notable is the absence of nickel in the PRE formula, given that in most cases it does not contribute to the evasion of pitting corrosion. 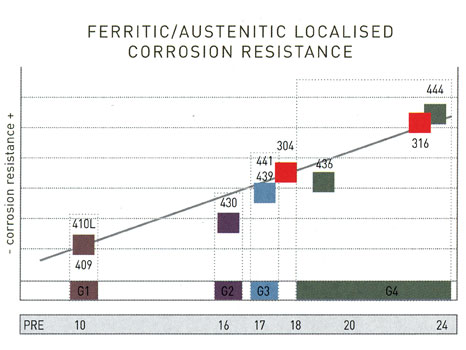
5. PRE comparison between popular and austenitic grades.
Stress corrosion cracking is a brittle failure mode caused by the combined effect of tensile stress and a corrosive environment. Like pitting and crevice corrosion, stress corrosion of stainless steels is most frequently caused by solutions containing chloride or very alkaline solutions. However, most cases of stress corrosion cracking on stainless steels occur at high temperature. At elevated temperatures, solutions that are unlikely to cause pitting and crevice corrosion, due to their low concentrations of chlorides and oxidizing chemicals, may give rise to stress corrosion.
Depending on the environment, relatively low engineering loads may provoke stress corrosion. Residual stresses from different manufacturing operations, such as forming and welding, can be high enough to cause failure. In some practical situations annealing at an appropriate temperature can reduce this potential risk, but such an approach is often difficult for large constructions. Coarse grinding induces tensile stresses into the steel surface that may facilitate initiation of stress corrosion.
Most cases of stress corrosion occur at temperatures above 50℃ but failures at ambient temperature have occurred on standard grade austenitic steels, such as 304 and 316 in swimming pool atmospheres.
Stress corrosion attack on stainless steel typically takes the form of thin, branched cracks. Failure caused by stress corrosion cracking often happens abruptly and without warning, due to the high propagation rates of the cracks. A common cause of stress corrosion is the concentration of chlorides from evaporation on hot steel surfaces. The evaporating liquid might be freshwater or other very dilute solutions, which because of their low chloride concentrations are considered as harmless. When the water evaporates, the chloride concentrations of these liquids might, however, become high enough to cause stress corrosion. 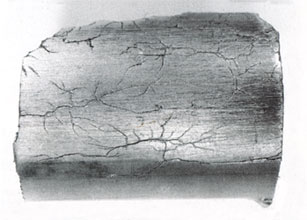
6. Stress corrosion cracking in a stainless steel tube.
Steels with a ferritic or duplex (ferritic-austenitic) structure generally display a very high resistance to chloride-induced stress corrosion attack. Standard austenitic grades, such as 304 and 316, are rather sensitive to this kind of corrosion. High contents of nickel and molybdenum increase the resistance of austenitic grades to stress corrosion and for this reason the grades (EN) 1.4539, 1.4547, 1.4565, 1.4652 show excellent resistance to chloride-induced stress corrosion cracking.
It is well known that a material that is subjected to a cyclic load can fail at loads far below the ultimate tensile stress of the material. If the material is simultaneously exposed to a corrosive environment, failure may take place at even lower loads and after shorter times. This is caused by a form of corrosion known as corrosion fatigue, which has similarities to stress corrosion cracking. Both corrosion forms cause brittle failures. Corrosion fatigue often takes place at ambient temperature and in moderately concentrated solutions that could be considered harmless with regard to other forms of corrosion.
Residual stresses from manufacturing can adversely affect resistance to corrosion fatigue. These may be reduced by an appropriate heat treatment or by the introduction of compressive stresses in the surface, as achieved by shot peening. High mechanical strength increases the resistance of a stainless steel to corrosion fatigue. Duplex steels are thus often superior to conventional austenitic steels. One application where a change from austenitic to duplex steels has reduced the number of failures caused by corrosion fatigue is for suction roll shells in paper machines. 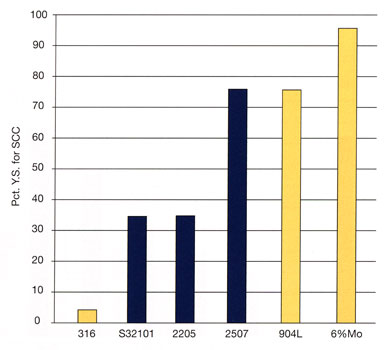
7. Stress corrosion cracking resistance of mill annealed austenitic and duplex stainless steels in the drop evaporation test with sodium chloride solutions at 120°C (stress that caused cracking shown as a percentage of yield strength).
Intergranular corrosion occurs when austenitic stainless steels are exposed to temperatures in the range 550-850°C where chromium carbides (Fe, Cr)23 C6 can precipitate in the grain boundaries. The chromium content of the carbide precipitates is very high, and since the diffusion rate of chromium in the austenite is low, the alloy adjacent to the grain boundary becomes chromium depleted. Since chromium is essential to passivity, the chromium-depleted region becomes less corrosion resistant than the matrix. In a corrosive environment the depleted area may be depassivated and corrosion will take place in very narrow areas along the grain boundaries. A stainless steel, which has been heat-treated in a way that produces grain boundary precipitates and adjacent chromium depleted zones, is said to be “sensitized”. Sensitization might occur as a result of welding, or of hot forming at an inappropriate temperature.
Measures to increase the resistance of stainless steels to intergranular corrosion caused by chromium carbide precipitation are solution annealing, lowering the carbon content and alloying with titanium or niobium (stabilizing).
Stainless steels are usually delivered from the steel producer in the solution-annealed condition. Solution annealing means heating to temperatures in the range 1000 – 1200°C, at which chromium carbides are dissolved, followed by rapid cooling in air or water. Such an operation leaves the carbon in solid solution in the steel. Steel with a sensitized structure may be recovered by a further solution annealing procedure. A low carbon content (< 0.03%) extends the time required for significant sensitization and is the second measure to decrease the risk of intergranular corrosion. Modern steel making methods enable much lower carbon contents to be achieved in steels than in the past (304L, 316L). 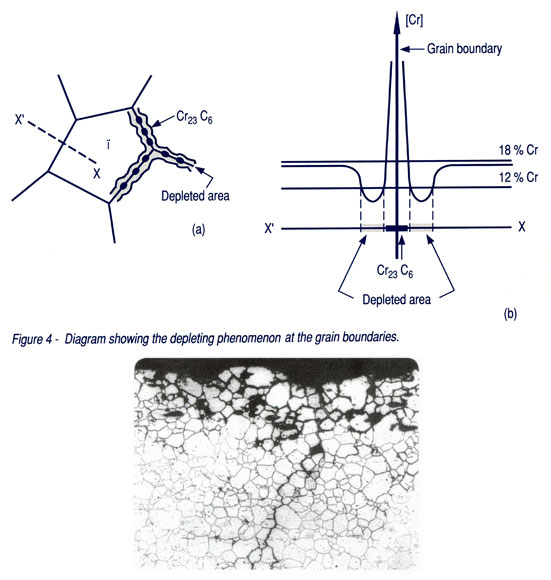
8. Intergranular corrosion attack.
Stabilized steels, containing titanium or niobium, show good resistance to intergranular corrosion even though their carbon contents may be fairly high. This is due to the fact that titanium and niobium form carbides more easily than chromium. Such carbides precipitate randomly in the grains and no carbon is available to form chromium carbide precipitates in the grain boundaries. Low carbon grades and stabilized grades can be considered as equally resistant to intergranular corrosion, unless exposed for long periods at temperatures above 500°C, in which case stabilized grades are to be preferred.
When two dissimilar materials are connected electrically and immersed in a conductive liquid, an electrolyte, their corrosion performance might differ significantly when compared with the uncoupled metals. As a rule, the less noble material (chemically active), the anode, is more severely attacked, whilst the more noble metal (chemically inert), the cathode, is essentially protected from corrosion. The corrosion attack is normally most evident close to the junction of the two metals. This phenomenon is called galvanic corrosion.
The degree of galvanic effects, as described above, will depend largely on the nature and kinetics of the electrochemical reactions taking place on the surfaces of the two materials forming a galvanic couple. Other factors that influence galvanic corrosion are the difference in the nobility of the two metals, the surface area ratio between the two metals and the conductivity of the solution.
The relative nobility of different conducting materials in a certain environment is indicated by the so-called galvanic series. Such series are valid for one specific environment and are based on measurements of the open circuit potential (OCP) of each material, uncoupled, in that environment. The higher the OCP, the more noble the material. The smaller the difference in OCP between the two metals forming a galvanic couple, the lower the driving force for galvanic attack. It should be noted that changes in electrolyte composition and temperature could cause significant changes in the positioning of different materials in a galvanic series. As long as stainless steels stay passive, they are in most environments nobler than other metallic construction materials and thus form the cathode in most galvanic couples. Galvanic coupling to stainless steels might, on the other hand, increase the corrosion rates of less noble metals such as mild steel, galvanized steel, copper and brass. Galvanic corrosion between different grades of stainless steel is generally not a problem, provided that each grade would be passive if exposed uncoupled in the particular environment. Galvanic corrosion can be prevented by the use of insulated flanges and isolation spools, but such insulators may cause crevice corrosion in chloride solutions.
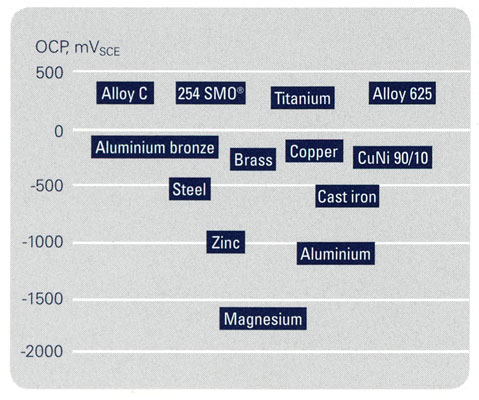
9. Galvanic series in flowing seawater, 10°C.
A small anode-to-cathode surface area ratio causes an increased corrosion rate of the anode and should thus be avoided. The coating or painting of a less noble material, galvanically coupled to an uncoated stainless steel, should also be avoided, as very high corrosion rates could be obtained on small anodic areas formed where coating defects occur. Coating the more noble metal in a galvanic couple is, on the other hand, an effective way to reduce the risk of galvanic attack.
The conductivity of the electrolyte affects the intensity as well as the location of the attack. A low conductivity tends to reduce the corrosion rate, but the attack can become very concentrated at the area adjacent to the contact site between the two metals.
When stainless steels are exposed to hot gases, chemical reactions take place between the steels, or rather between their alloying elements, and elements or compounds in the gases. As a rule, these reactions will lead to the formation of gas metal interface layers of reaction products. These surface layers are more or less protective against further attack from the gas. In practice, all stainless steels rely on the formation of an oxide layer. All other reaction products either form a porous, less adherent, and hence non-protective scale or are liquid or even volatile and will flux off any existing scale or evaporate. Thus, one condition that must be fulfilled for good high temperature corrosion resistance is that the gas must be oxidizing, i.e. it must contain enough oxygen for the formation of a protective layer, consisting of oxides of one or several of the alloying elements.
There exist several types of high temperature corrosion (oxidation, sulphidation, carburisation and nitridation, furnace gases, etc) depending on the temperature’s level, the time the steel is exposed, the gases’ composition etc. When choosing material for high temperature applications, one must have an extensive knowledge of existing or expected service conditions, such as gas temperature and composition, and material temperature. After consultation with a reputable supplier, one may come to choose the most suitable type of stainless steel for each corresponding high temperature environment.
Atmospheric corrosion is not a unique form of corrosion, but a collective term to denote the corrosion of surfaces in the atmosphere. It may be indoor or outdoor and all corrosion forms can, in principle, be involved. Corrosion of stainless steels is most often induced by halides and mainly chlorides due to their abundance in our environment. When stainless steel is exposed to an aggressive atmosphere it is primarily stained (sometimes referred to as tea staining), but it can also be attacked by localized corrosion with time, particularly at high chloride levels. It should be noted that discoloration of stainless steel is not automatically the result of atmospheric corrosion or even corrosion attack at all; it can be a discoloration from dirt or extraneous rust, not affecting the stainless steel.
Two large application areas can be distinguished where implementation of atmospheric corrosion knowledge is needed: in architectural and structural use. The surface appearance is much more important for architectural use, while superficial corrosion attack can be neglected in structural applications.
Since the corrosion process in the atmosphere is based on wet corrosion it needs a conductive electrolyte for charge transport and dissolution of metal atoms. Therefore, temperature, relative humidity and weather conditions affect the corrosion process on the stainless steel surface, as do polluting gases (i.e. SO2) and other contaminants and aggressive substances that can dissolve into the electrolyte. Moreover, the exposed condition of the stainless steel’s surface is of great importance: sheltering prevents rinsing by rainfall, thus increasing the corrosivity of the environment, and the inclination angle affects the run-off rate. Additional crucial factors are the surface condition and its smoothness. A coarse surface will retain dirt, particles and corrosive chemicals while a smoother surface (BA and 2B) facilitates rinse-off. Finally, one has to take into account that regular cleaning acts decisively for maintaining the metal in ideal condition. 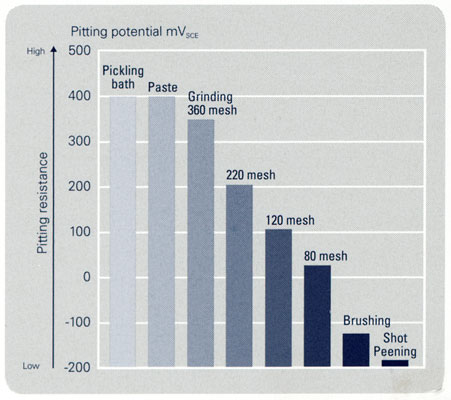
10. Relative pitting corrosion resistance of welded 4401 (316) after different post weld cleaning treatments.
Below there are three tables that present the suitability of various popular stainless steel grades to different environments.
| Typical indoor environments | Typical outdoor environments | Suitable stainless steel grades |
| Heated | Deserts and artic areas (rural) | 4016, 4521, 4301, 4372, 2101 |
| Non – heated | Dry environment with low pollution | 4016, 4521, 4301, 4372, 2101 |
| Humid environment with low pollution | Coastal environment with low salt concentrations, urban and industrial areas with moderate pollution | 4521, 4301, 4401, 2101, 2304 |
| Polluted urban and industrial environments, coastal areas with moderate salt deposits | 4401, 4438, 4439, 904L, 254 SMO, 4565, 2304, 2205, 2507 | |
| Swimming pool environments, humid industrial areas with (volatile) aggressive chemicals | Heavily polluted industrial environments, marine atmosphere with high salt concentrations in the atmosphere and a combination of marine and industrial environments | 904L, 254 SMO, 4565, 2205, 2507 |
11. Material selection sheet for some ferritic, austenitic and duplex grades in four different environments: rural, urban, industrial and marine with three different corrosion categories, low (L), medium (M) and high (H), respectively.
Type of outdoor environment and corrosion category
Steel grade |
Rural L M H |
Urban L M H |
Industrial L M H |
Marine L M H |
| 1.4003 1.4016 / 430 1.4521 / 444 | o x x + o o - - - | x x x x x x - + o | x x x x x x + o x | x x x x x x + o o |
| 1.4301 / 304 1.4401 / 316 1.4439 / 317 LMN 1.4539 / 904L | + + + - - - - - - - - - | + + o - + + - - - - - - | o o x + + o - - + - - + | + o x + + o - - + - - + |
| 1.4162 / 2101 1.4362 / 2304 1.4462 / 2205 | - - + - - - - - - | - + o - + + - - - | + o x + + o - - + | + o x + + o - - + |
+ = optimum choice, o = may be used with precautions, x = not suitable, - = over-specified 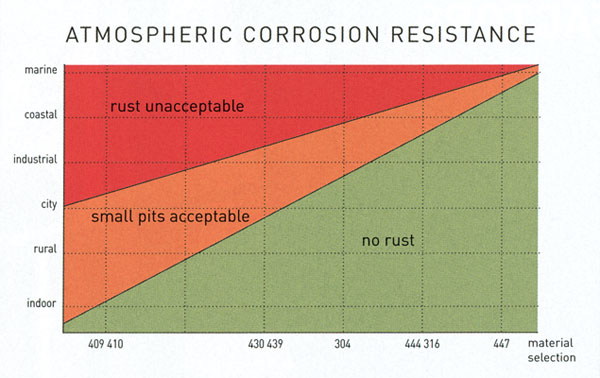
The generally held view for stainless steels is characterized by some erroneous conceptions that can easily be attributed to widespread ignorance. One misconception is that stainless steel does not have iron, when this is actually its primary constituent (around 70 %). Another incorrect view is the one that considers stainless steel (usually grade 304) as a solution to any kind of corrosion, and thus when a corrosion issue arises, then people accuse the steel that it is not stainless. However, we saw earlier that we have the possibility of choosing among a great variety of stainless steel grades. What one must do is the study and understanding of the conditions prevailing during the material's application and functioning and the consultation with a credible supplier. At the same time, the final user must certainly take a decision for the relation he accepts between the material's cost and its life cycle (there are "everlasting" types that yet cost dearly). Of utmost importance for preserving stainless steel in good condition is its regular cleaning, as well as the procedure of chemical cleaning (pickling/passivation) Chemical cleaning refers to the procedures of pickling and passivation. Pickling is the removal of a thin layer of metal from the surface of the stainless steel. Mixtures of nitric and hydrofluoric acids are usually used for pickling stainless steels. Passivation usually occurs naturally on the surfaces of stainless steels, but it may sometimes be necessary to assist the process with oxidizing acid treatments. Solutions of nitric or citric acid are used for passivating stainless steel surfaces. If the metal's surface is contaminated with grease, oil or inorganic contaminants, then a cleaning operation prior to acid treatment should be carried out. The two operations can be applied to all stainless steel types. with the completion of the construction.
Finally, a widely held misconception is that because ferritic and duplex types are magnetic they are not real stainless steels and will rust like carbon steel. This is wrong. Purely for reasons of atomic structure, some stainless steels are magnetic and some are not. Corrosion resistance is not a matter of atomic structure but one of chemical composition. Magnetism has nothing to do with it. Reference was made earlier to the PRE formula which is used to state each type's resistance to pitting corrosion. Studying the PRE number of both magnetic and non-magnetic types we easily find out that there exist magnetic grades with higher PRE, and thus move resistant, to non-magnetic. Below we present a table with the PRE number of the most popular grades.
Typical chemical composition, % by wt.
| AISI | EN | Cr | Ni | Mo | N | others | PRE |
| ferritic | stainless steels | - | |||||
| 430 | 1.4016 | 16,5 | - | - | - | 17 | |
| 441 | 1.4509 | 18 | - | - | - | Nb, Ti | 18 |
| 444 | 1.4521 | 17,8 | - | 2.1 | 0.01 | Ti | 25 |
| austenitic | stainless steels | ||||||
| 304L | 1.4307 | 18,1 | 8.1 | - | - | - | 18 |
| 316L | 1.4404 | 17,2 | 10.1 | 2.1 | - | - | 24 |
| 904L | 1.4539 | 20 | 25 | 4.3 | - | 1.5 Cu | 34 |
| duplex | stainless steels | ||||||
| S32101 | 1.4162 | 21,5 | 1.5 | 0.3 | 0.22 | 5Mn | 26 |
| S32304 | 1.4362 | 23 | 4.8 | 0.3 | 0.10 | - | 26 |
| S32205 | 1.4462 | 22 | 5.7 | 3.1 | 0.17 | - | 35 |
PRE = % Cr + 3.3 x % Mo + 16 x % N
13. 
14.
Concluding, stainless steel's main properties can be summarized as follows
corrosion resistance
aesthetic appeal
heat resistance
low lifecycle and maintenance cost
full recyclability
biological neutrality (meets EU RoHS requirements)
ease of fabrication and durability
Alloy is the material that is composed from various chemical elements. When it is solid, it is characterized by the participation of all the elements into the crystal structure. That is, the material is crystalline and in this crystal the atoms of its constituents are arranged in space as if they are atoms of the same kind. The crystal structure reflects the geometrical construction of a chemical element. It is called "crystal" in the case of a solid material that exhibits regular geometrical arrangement of its structural constituents. Depending on the geometrical arrangement the atoms or other particles of the element form, the crystal structure is distinguished into seven lattice systems: triclinic, monoclinic, orthorhombic, rhombohedral, tetragonal, hexagonal and cubic.
Annealing see "heat treatments"
Atom see "chemical element"
Carbide is a chemical compound that consists of carbon and a less electronegative element. With the term "electronegative" we describe the chemical property of the tendency an atom has to attract electrons. The highest the value of this property is, the more the element draws electrons towards itself.
Chemical cleaning refers to the procedures of pickling and passivation. Pickling is the removal of a thin layer of metal from the surface of the stainless steel. Mixtures of nitric and hydrofluoric acids are usually used for pickling stainless steels. Passivation usually occurs naturally on the surfaces of stainless steels, but it may sometimes be necessary to assist the process with oxidizing acid treatments. Solutions of nitric or citric acid are used for passivating stainless steel surfaces. If the metal's surface is contaminated with grease, oil or inorganic contaminants, then a cleaning operation prior to acid treatment should be carried out. The two operations can be applied to all stainless steel types.
Chemical element is a chemical substance that cannot change or be divided into simpler forms. The atom is its smallest unit. The atoms consists of Protons - one element's atoms have the same number of protons. They are positively charged and together with the neutrons (that do not have any charge) they constitute the atom's nucleus and are called nucleons Electrons - electronic cloud that surrounds the nucleus with negative charge and smaller mass. The number of the atom's protons that determines the kind of the element is called the atomic number (Z) and constitutes the element's identity number. All the atoms of an element have the same atomic number. The sum of the protons and the neutrons that exist in an atom's nucleus is called the mass number (A).
The atoms are electrically neutral when they have the same number of protons and electrons. The electrons have the capability of moving to neighboring atoms or being shared among them. Atoms that experience deficit or surplus of electrons are named as ions and carry electric charge. When the atoms acquire electrons, then they form ions negatively charged and are called anions. On the contrary, when they lose electrons they turn into cations, with positive electric charge. When the atoms form chemical compounds they seek to acquire the least possible energy. This takes place with the following chemical bonds. COVALENT BOND is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. NON COVALENT BOND is the exchange of electrons with other atoms.
The electrons' number at the outermost electron shell (cloud) of the atom governs its chemical bonding behavior. These are called valence electrons and are those that can participate in the formation of chemical bonds with other atoms. Valence electrons can determine the element's chemical properties and if it will bond with others or not. Valence electrons are important in determining how the atom reacts chemically with other atoms. Atoms with a complete (closed) shell of valence electrons tend to be chemically inert (i.e. noble gases as opposed to alkali metals that have only one electron - for example sodium and potassium). Atoms with one or two valence electrons more than a closed shell are highly reactive because the extra electrons are easily removed to form positive ions. Atoms with one or two valence electrons fewer than a closed shell (i.e. halogens - for example fluorine and chlorine) are also highly reactive because of a tendency either to gain the missing electrons and form negative ions, or to share electrons and form covalent bonds. The number of valence electrons of an element is determined by its periodic table group in which the element is categorized.
The periodic table is a tabular display of the chemical elements, organized on the basis of their properties. It accurately predicts the properties of various elements and the relations between properties. As a result, it provides a useful framework for analyzing chemical behavior, and is widely used in chemistry and other sciences. Elements are presented in increasing atomic number. The table classifies the elements according to their similarities and differences. In this table, the elements are presented in vertical columns (groups) and horizontal rows (periods) in consecutive number of their atomic number. The position and the properties of each element are determined by the number of the valence electrons at the outermost shell of their atoms. Therefore, the elements found in the same column of the periodic table have the same number of valence electrons and thus similar chemical properties. While the elements found in the same row (period) allocate their electrons on the same shell number (in their basic condition), presenting as a result similar physical properties. As chemical properties we refer to the characteristics of an element that influence its behavior when it reacts with other elements under any conditions and are dependent on its chemical composition. Physical properties are the general features of a material in its natural form. That is, the state in which the material exists (solid, liquid, gas), its temperature, size etc. The physical properties are not dependent on the element's chemical composition.
We note that the main alloying elements that are present in stainless steels are near to each other in the periodic table, having similar atomic number (Ti 22, Cr 24, Mn 25, Fe 26, Ni 28).
Heat treatments are a group of industrial and metalworking processes used to alter intentionally the steel's microstructure with the aim of shaping its properties. Heat treatment involves the use of heating or chilling, normally to extreme temperatures, to achieve a desired result such as hardening or softening of a material. Annealing is the most important heat-treating process. It consists of heating a metal to a specific temperature and then cooling at a rate what will produce a refined microstructure. Annealing is most often used to soften a metal for cold working, to improve machinability, or to enhance properties like electrical conductivity. Annealing includes the heating of the material in specific temperature and for specific time interval, and then cooling slowly in room temperature. It is used to remove the hardness caused by cold working and to repair the defects caused by plastic deformation. Annealing is distinguished to:
Full annealing which aims to soften the steel before any machine working. The cooling takes place at very slow rates, usually inside an oven.
Normalizing aims at the homogeneity of the alloying elements for achieving the best transformation of the austenite during cooling. It includes heating in the austenite region with temperatures higher than those applied in full annealing. The metal is then cooled in open air. It is a technique used to provide uniformity in grain size and composition throughout an alloy. Normalizing gives harder and stronger steel, but with less ductility for the same composition, than full annealing.
Stress relief annealing is a technique to remove or reduce the internal stresses created in a metal. These stresses may be caused in a number of ways, ranging from cold working to non-uniform cooling. Stress relieving is usually accomplished by heating a metal below the lower critical temperature and then cooling uniformly.
Heat treatments are also used for the strengthening of steel. A classical heat-treating method for this purpose is quenching and tempering. After the steel's austenization in high temperature, where the carbides of the alloying elements are dissolved, the steel is subjected to quenching by a medium (air or other gases, oil or water) depending on the alloy and other considerations (such as concern for maximum hardness vs. cracking and distortion). Therefore, by being rapidly cooled, a portion of austenite (dependent on alloy composition) will transform to martensite, a hard, brittle crystalline structure. The quenched hardness of a metal depends on its composition and quenching method. Cooling speeds, from fastest to slowest, go from fresh water, oil and forced air. Quenching is followed by tempering as untempered martensitic steel, while very hard, is too brittle to be used for most applications. Tempering is the method used for alleviating this problem and it consists of heating a steel below the lower critical temperature (often from 205 to 595℃ depending on the desired results), to impart some toughness.
Finally, we note the presence of surface treatments that affect only the surface of an engineering material (i.e. carburisation and nitridation which describe the enrichment with carbon and nitrogen for higher surface hardness).
Ion see "chemical element"
Iron the chemical element symbolized by Fe is a metal with atomic number 26, melting and boiling temperatures 1535℃ and 2750℃ respectively. It is the forth most abundant element on the earth's crust after oxygen (O), silicon (Si), and aluminum (Al). Moreover, it is the metal with the widest use, especially in the form of its two most important alloys, that of steel and that of cast-iron (iron alloy with carbon C > 2,1 %).
Mechanical properties of a metallic alloy are those that describe the material's ability to compress, stretch, bend, scratch, dent or break. The most commonly used criteria for evaluating mechanical characteristics are: a. Strength: the degree of resistance of a material to deformation. Two critical values are generally considered: yield strength, or the stress the material can be subjected to before permanent plastic deformation occurs tensile strength, or the stress it can be subjected to before rupture/failure 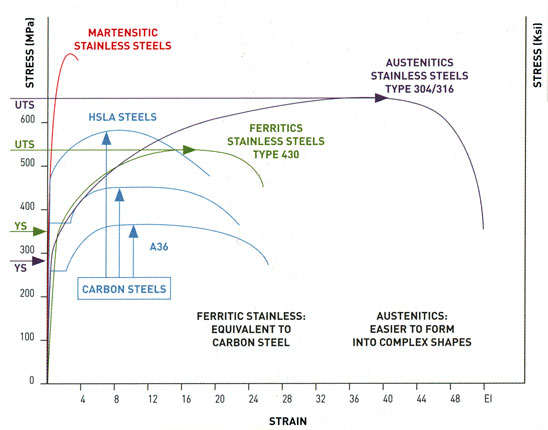
15. UTS (ultimate tensile strength) is measured in MPa (1Mpa=1N/mm3=145PSI=0.1 kg/mm3) and represents maximum resistance at failure. YS (yield strength) refers to the beginning of the "plastic" phase, where elongation no longer disappears when the stress is removed.
b. Hardness: the degree of resistance to indentation by an applied load. c. Toughness: the capacity to absorb deformation energy before fracture. d. Ductility (or plasticity): the ability to deform plastically without fracturing. 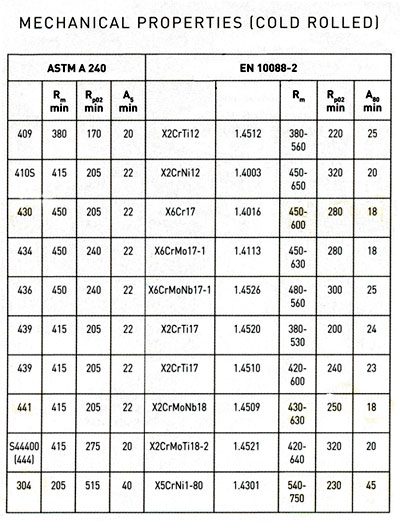
16. Rm=ultimate tensile strength, Rp02=yield strength and A5/A80=elongation to fracture.
Metal is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny. Most of them have high density and are hard and tough (iron, copper, aluminum, sodium, calcium, zinc, magnesium, titanium, uranium, etc). Except from mercury, they are all solids in room temperature 20℃. In a metal, atoms readily lose electrons to form positive ions (cations). Those ions are surrounded by de-localized electrons, which are responsible for the electrical conductivity. The solid thus produced is held by electrostatic interactions between the ions and the electron cloud, which are called metallic bonds. The metals are extracted from minerals through a series of procedures described by the term of metallurgy. Apart from metals, there exist two more important categories of chemical elements: the non-metallic and the metalloids.
Microstructure is defined as the structure of a prepared surface or thin foil of material as revealed by a microscope above 25 X magnification. The microstructure of a material (which can be broadly classified into metallic, polymeric, ceramic and composite) can strongly influence its properties such as strength, toughness, ductility, hardness, corrosion resistance, high/low temperature behavior, wear resistance, and so on, which in turn govern the application of these materials in industrial practice. 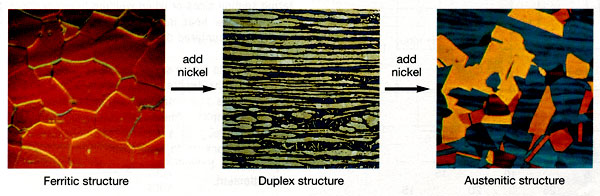
17. Increasing the nickel content changes the microstructure of the stainless steels.
Mineral is a naturally occurring solid chemical substance formed through biogeochemical processes, having characteristic chemical composition, highly ordered atomic structure (usually crystalline), and specific physical properties. By comparison, a rock is an aggregate of mineral and/or mineraloids and does not have a specific chemical composition.
pH is a way of expressing the concentration of hydrogen's ions into a liquid solution. With pH we symbolize the negative decimal logarithm of the hydrogen ions' [H+] concentration in the solution pH = -log[H+]. The pH level is a unit of measure describing the degree of acidity or alkalinity of a solution. This is measured on a scale of 0 to 14.
pH < 7 - acid solutions (i.e. coffee, vinegar, lemon juice, gastric fluids)
pH > 7 - alkaline solutions (i.e. saliva, blood, seawater, chlorine)
pH = 7 - neutral solutions (pure water)
Phase in the physical sciences is a region of space (a thermodynamic system), through out which all physical properties of a material are essentially uniform. A simple description is that a phase is a region of material that is chemically uniform, physically distinct and often mechanically separable. A material's transition from one phase to another has to do with changes in its state (i.e. from solid to liquid) or more imperceptible changes like those related to its crystal structure.
Physical properties of a metallic alloy concern the material's ability to conduct heat, conduct electricity, expand or shrink. 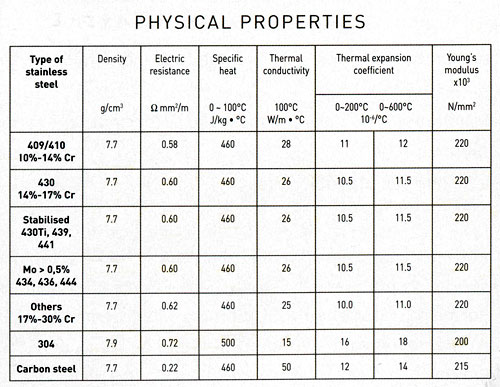
18. Physical properties
Solid solution is a solid-state solution of one or more solutes in a solvent. Such a mixture is considered a solution rather than a compound when the crystal structure of the solvent remains unchanged by addition of the solutes, and when the mixture remains in a single homogeneous phase. This often happens when the two elements (generally metals) involved are close together on the periodic table. Conversely, a chemical compound is generally a result of the non-proximity of the two metals involved on the periodic table. Stabilizer is an element that prevents carbide formation, primarily along the grain boundaries. To this purpose, stainless steel is alloyed with titanium and niobium. These stabilizing elements preferentially combine with the excess carbon in the steel, thereby leaving the chromium to perform its function of forming and maintaining a stable oxide layer (passive film). Tensile strength see "mechanical properties" Yield strength see "mechanical properties"
Steels are the iron alloys with 2% maximum carbon content that constitute over the 80% of the industrial alloysAlloy is the material that is composed from various chemical elements. When it is solid, it is characterized by the participation of all the elements into the crystal structure. That is, the material is crystalline and in this crystal the atoms of its constituents are arranged in space as if they are atoms of the same kind. The crystal structure reflects the geometrical construction of a chemical element. It is called “crystal” in the case of a solid material that exhibits regular geometrical arrangement of its structural constituents. Depending on the geometrical arrangement the atoms or other particles of the element form, the crystal structure is distinguished into seven lattice systems: triclinic, monoclinic, orthorhombic, rhombohedral, tetragonal, hexagonal and cubic.. The latter is attributable to their low cost of production and the relative ease of producing steel in large quantities with accurate standards - specifications. The following three factors play a determinant role in the widespread use of steel.
(a) the high global reserves of mineralMineral is a naturally occurring solid chemical substance formed through biogeochemical processes, having characteristic chemical composition, highly ordered atomic structure (usually crystalline), and specific physical properties. By comparison, a rock is an aggregate of mineral and/or mineraloids and does not have a specific chemical composition. (the earth crust consists of around 4% iron FeIron the chemical element symbolized by Fe is a metal with atomic number 26, melting and boiling temperatures 1535°C and 2750°C respectively. It is the forth most abundant element on the earth’s crust after oxygen (O), silicon (Si), and aluminum (Al). Moreover, it is the metal with the widest use, especially in the form of its two most important alloys, that of steel and that of cast-iron (iron alloy with carbon °C > 2,1 %).) which can be easily converted into the metallic condition, together with the availability coming from the recycling of scrap.
(b) the iron’s melting point (1539°C) allows the thermal activation of processes at temperatures (Τ.>400°C) which can be achieved relatively easily and be controlled by industrial manufacturing.
(c) the allotropy of iron and the transformation of steel’s phases (i.e. martencitic transformation) allow the formation of a great variety of microstructures which leads in turn to a respectively large range of mechanical properties. Elements that are characterized by the condition of allotropy are those that appear with more than one natural forms, due to the fact that their atoms are matched with one another in various ways (i.e. graphite and diamond constitute manifestation for the allotropy of carbon).
All the above-mentioned, make steel as the most important and popular material in mechanical engineering. Much of this stems from the allotropy of iron. The formation of the structure and the properties shown by different types of steel are achieved through industrial procedures named as heat treatments (most known being that of annealing). The greatest variety of steel microstructures appear during the transformation of austenite while it is being cooled. So, according to the allotropy of iron, we have the phase of α-Fe BCC (body centered cubic crystal lattice) dominating up to 910°C, the phase of γ- Fe FCC (face centered cubic crystal lattice) between 910 and 1400°C and α-Fe reappearing from 1400°C and to the melting point.

1. By adding nickel, the crystallographic structure changes from body-centered cubic (little or no nickel) to face-centered cubic (at least 6% nickel – 300 series).
The solid solution α-Fe with carbon is named ferrite, while the corresponding solid solution γ-Fe with carbon, austenite. Main difference between the two phases is the solubility of carbon which is much higher in the austenite than the ferrite. For example, the austenite can dissolve 2% carbon while the ferrite just 0,02% by weight.
Even though the most important alloying element in steel is carbon, in most cases several other alloying elements are added for various reasons. Thus, in most types of steel we will observe the presence of Mn and Si as well as that of Cr, Ni and Mo. The role of an alloying element is compound. It influences the solid solubility of the other alloying elements, the thermodynamic stability of the phases and in general, the formation of the steel’s microstructure together with its physical and mechanical properties. More specifically, the elements are distinguished into two categories according to their tendency to promote either the austenitic or the ferritic microstructure. The most important impacts of the alloying elements are mentioned below in brief.
* The present text has been registered at a notary office for the intellectual rights' protection.
Chromium (Cr) is a highly carbide forming element. Chromium carbides raise the steel’ s toughness and increase scaling and wear resistance. Moreover, chromium increases the resistance to oxidation (including that caused by high temperatures) and the resistance to corrosion. It constitutes the main alloying element for stainless steel.
Molybdenum (Μο) is a strong carbide former. It gives steel extra resistance to corrosion and particularly to pitting and crevice corrosion – these terms are explained later. The high melting point of molybdenum makes it important for giving strength to steel at high temperatures.
Titanium (Ti) and Niobium (Nb) are both elements that form carbides and raise steel’s strength and resistance – even at high temperatures. Both of them play the role of stabilizer and contribute in reducing the risk of intergranular corrosion – it is explained later.
Silicon (Si) and Aluminum (Al) are two elements used for the deoxidization of steel. High silicon content leads to lower ductility. Both of them increase resistance to oxidation in high temperature environments.
Nickel (Ni) in stainless steels has no direct influence on corrosion resistance. Thanks to nickel austenitic stainless steels exhibit a wide range of ideal mechanical properties, like excellent ductility and toughness, even at high strength levels. These properties are retained up to cryogenic temperatures.
Carbon (C) is an important alloying element in all-ferrous metal based materials. Carbon is a very strong austenitizer and increases the strength of steel. By increasing its content we raise hardness and toughness and make the steel heat treatable by quenching and tempering to develop the martensite phase. However, resistance to fracture, ductility and weldability are reduced with higher carbon content.
Nitrogen (N) raises the strength and the resistance of steel, while at the same time it reduces its ductility. More specifically, it improves the mechanical properties of austenitic and duplex stainless steels, increasing at the same time their resistance to localized corrosion like pitting or intergranular.
Manganese (Mn) is essential to steel making because of two key properties: its ability to combine with sulphur and its powerful deoxidation capacity. Manganese will combine preferentially with sulphur to form manganese sulphide (Mn S) which favour workability and weldability of steel. Moreover, its presence increases the hardenability of the steel. Its ability to stabilize the austenite in steel is used to substitute nickel in some austenitic stainless steels.
Copper (Cu) improves resistance to corrosion from sulphuric acid.
Neutral elements
Sulphur (S) and Phosphorous (P) are undesired impurities. They act on reducing ductility, weldability. At the same time they reduce resistance to corrosion and increase steel’s inclination to cracking.
Stainless steel is an alloy of iron, carbon and chromium with minimum content of chromium at least 10,5 % by weight. Chromium creates an adherent, insoluble film on the surface of the steel that shields the metal substrate from uniform and localized attack. This protective film is called passive layer or passive film and constitutes a very fine layer on the surface of the steel (its thickness is 5-15 nm). Apart from chromium, stainless steels may contain and other alloying elements like nickel Ni, molybdenum Mo, manganese Mn etc (we examined them earlier).
They are widely used into several applications that require among others resistance to corrosion, effective relation between cost and life cycle, for their aesthetic or hygienic properties. Stainless steels apart from their resistance to corrosion, they also enjoy higher mechanical properties in comparison with other common types of steel. Yet, they are harder and more difficult to work with. An additional characteristic is their lower thermal conductivity.
Stainless steels are distinguished according to the dominant phase in their crystal lattice.
Austenitic stainless steels They are stainless steels with main phase that of the austenite (γ-Fe). Austenite is the allotropic form of iron, which crystallizes according to the face centered cubic system (FCC). Austenitic stainless steels contain very low quantity of carbon (usually <0,08% C, but some contain up to 0,15% C) and at least 16% Cr. The austenite is stabilized with the addition of Ni or and Mn, and remains into stable phase in all the temperature breadth from the alloy’s melting point until well below 0℃. Due to the austenite’s non-magnetic nature, austenitic stainless steels are not magnetic.
The most common austenitic stainless steels are 18/8 (18% Cr, 8% Ni) and 18/10 (18% Cr, 10% Ni), which belong in 300 series, according to the American standards AISI-SAE. The steels AISI 316 display higher resistance to corrosion and are characterized by the presence of molybdenum (around 2%). The types 304L and 316L contain the lowest quantity of carbon (< 0,03%) resulting in better performance during welding. Generally, the types of the 300 series present good corrosion resistance, excellent forming capability, low yield strength Mechanical properties of a metallic alloy are those that describe the material’s ability to compress, stretch, bend, scratch, dent or break. The most commonly used criteria for evaluating mechanical characteristics are:
a. Strength: the degree of resistance of a material to deformation. Two critical values are generally considered:
yield strength, or the stress the material can be subjected to before permanent plastic deformation occurs
tensile strength, or the stress it can be subjected to before rupture/failure
15. UTS (ultimate tensile strength) is measured in MPa (1Mpa=1N/mm3=145PSI=0.1 kg/mm3) and represents maximum resistance at failure. YS (yield strength) refers to the beginning of the “plastic” phase, where elongation no longer disappears when the stress is removed.
b. Hardness: the degree of resistance to indentation by an applied load.
c. Toughness: the capacity to absorb deformation energy before fracture.
d. Ductility (or plasticity): the ability to deform plastically without fracturing.
16. Rm=ultimate tensile strength, Rp02=yield strength and A5/A80=elongation to fracture. , relatively high tensile strength Mechanical properties of a metallic alloy are those that describe the material’s ability to compress, stretch, bend, scratch, dent or break. The most commonly used criteria for evaluating mechanical characteristics are:
a. Strength: the degree of resistance of a material to deformation. Two critical values are generally considered:
yield strength, or the stress the material can be subjected to before permanent plastic deformation occurs
tensile strength, or the stress it can be subjected to before rupture/failure
15. UTS (ultimate tensile strength) is measured in MPa (1Mpa=1N/mm3=145PSI=0.1 kg/mm3) and represents maximum resistance at failure. YS (yield strength) refers to the beginning of the “plastic” phase, where elongation no longer disappears when the stress is removed.
b. Hardness: the degree of resistance to indentation by an applied load.
c. Toughness: the capacity to absorb deformation energy before fracture.
d. Ductility (or plasticity): the ability to deform plastically without fracturing.
16. Rm=ultimate tensile strength, Rp02=yield strength and A5/A80=elongation to fracture. and good weldability, providing a wide range of applications.
Apart from the well-known 300 series, there exist the less resistant types of the 200 series with manganese. These new types use different chemistry which is differentiated by the lower chromium content (<15%) and the much lower nickel content. The reduction of nickel with the simultaneous addition of manganese limits the quantity of chromium that can be added, affecting negatively therefore the resistance to corrosion. It has been mentioned earlier that the addition of nickel is the principal way for protecting the austenitic structure in stainless steel. However, the addition of manganese in combination with the presence of nitrogen may bring about the same results – albeit at lower cost. The chromium–manganese types are characterized by notably lower nickel content and by the existence of manganese and often that of nitrogen and copper (both of which share the capability of promoting the austenitic structure). The addition of nitrogen results to a better stabilization of the austenitic phase, allowing therefore the addition of more chromium. Nitrogen also acts as a hardening factor. We use to refer to the types of the 200 series simply by mentioning their nickel content – like 4% Ni and 1% Ni. The most popular types are 201 (1% Ni, min 15% Cr, max 0,1% C) and 202 (4% Ni, min 16% Cr, max 0,08% C).
Apart from the fact that the chromium–manganese stainless steels have a lower cost, at the same time they present good forming capability and good corrosion resistance depending on their chemistry. Their characteristic advantage is their higher mechanical properties compared to those of the classical 300 series (i.e. 304), something that allows the reduction of the gauge of the material used and thus the reduction of the weight. However, we draw your attention to the fact that the 200 series (especially types with high nitrogen content) are more difficult to form. The addition of copper is a solution to this problem, since it allows the reduction of nitrogen with the content of nickel and chromium remaining stable.
The rise in the popularity of the 200 series was related to the instability of the nickel value (especially during periods of sharp increases in its price), as well as to the progress in the technology used for the steel’s production. At the same time, constant pressure for the reduction of cost, especially in the Asian market, has led to the development of austenitic types with low nickel and chromium content which often do not satisfy any international standards. Actually, several chromium–manganese types are distinctive of specific mills’ production and are defined simply from a title given by each producer. Therefore, it is suggested to manufactures that examine their potential use to take the advice of credible suppliers who have the right information and are capable of supplying good quality products of reliable origin. Moreover, we note that the potential user has the possibility to choose alternative solutions among the 400 and 300 series.
Concluding the 200 series presentation, we mention below some applications were the experience has proven their positive performance (mainly for the types with 4% Ni). Such applications include cutlery and cooking utensils, home sinks, catering equipment, trucks’ structural parts, bushes’ chassis and constructions in the sugar industry, as well as architectural constructions that are not near coasts.
Finally, we make a reference to the super austenitic stainless steels with very high nickel content Ni (>20%) and molybdenum Μο (>6%), for strong resistance to the corrosion caused by acids, chlorine and chloride solutions. The most popular type of this category is AISI 904L (19-23% Cr, 23-28% Ni, 4-5% Mo).
These are stainless steels with main phase that of ferrite (α-Fe) or martensite (phase that results from the rapid cooling of the austenite). They contain 10,5-27% chromium, with very little or none at all nickel (<2%). Some of them have molybdenum or titanium and niobium.
The martensitic stainless steels contain 12-17% chromium and have higher carbon content. These kinds of stainless steels are subjected to specific heat treatment that leads to an increase of their hardness. They are used for the manufacturing of turbine propellers, cutlery, knife blades etc.
The ferritic stainless steels share the same advantages (mechanical properties and corrosion resistance) with the austenitic types. In some cases their performance is superior to that of the austenitics. In short, we mention below the special advantages of the ferritic stainless steels.
Ferritic grades fall into five groups – three families of standard grades and two of "special" grades.
Group 1 (409/410L) has the lowest chromium content (10-14%) of all stainless steels and is also the least expensive. This group can be ideal for non–or lightly–corrosive environments or applications where slight localized rust is acceptable. Typical applications are automotive exhaust–system silencers for the type 409, while 410L is often used for containers, buses, coaches and LCD monitor frames.
Group 2 (430) is the most widely used family of ferritic alloys. Having a higher chromium content (14-18%), group 2 grades show greater resistance to corrosion and behave most like austenitic grade 304. Typical uses include washing–machine drums, household utensils, dishwashers, pots and pans and indoor panels. Generally speaking, we note the fact that 430 is suitable to replace 304 in some applications where the use of the latter is judged as an excessive (over-alloyed) and expensive choice (i.e. catering equipment).
Group 3 includes 430Ti, 439, 441. Compared with group 2, these grades show better weldability and formability. Their behavior is even, in most cases, better than that of 304 austenitic grades. This group has chromium content between 14 and 18% and is characterized by the presence of stabilizing elements like titanium and niobium. Sinks, exchanger tubes, exhaust systems (longer life than with type 409) and catering equipment are some of the typical applications. Group 3 grades can replace type 304 in applications where this grade is an over – specification. 
2. Τhe Limited Drawing Ratio (LDR) is an important deep-drawability parameter. It refers to the quotient of the maximum blank diameter (D) that can be deep drawn into a cylinder in one step and the diameter (d) of that cylinder. LDR=D/d.
Group 4 includes types 434, 436, 444, etc. These grades have added molybdenum, for extra corrosion resistance. The chromium content lies between 14 and 20%. Typical applications include hot water tanks, solar water heaters, visible parts of exhaust systems, electrical – kettle and microwave oven elements, automotive trim and outdoor panels, etc. Type 444’s corrosion resistance level can be similar to that of type 316.
Group 5 (types 446, 445/447 etc) has additional chromium (18-30%) and contains molybdenum, for extra corrosion and scaling (oxidation) resistance. The grades of this group are superior to type 316 in respect of these properties. Typical uses include applications in coastal and other highly corrosive environments.
Duplex (austenitic-ferritic) stainless steels are comprised by austenite and ferrite in proportions from 50:50 to 40:60. Usually, they contain 19-28% Cr, <5% Mo and a bit of Ni (1,5-7% depending on the grade). Thanks to their high content of chromium, nitrogen and molybdenum they show excellent corrosion resistance, enjoying at the same time the advantage of having higher mechanical properties in comparison to the other families.
For instance, the type S32101 has 90% higher mechanical properties than 304, allowing thus the safe reduction of the material’s thickness by 30% (on average).
On top of that, due to their low nickel content they have a more stable price than the austenitic stainless steels. They are distinguished into the following groups.
Metals, excluding precious metals such as gold or platinum, found in their natural state, are always extracted from minerals (oxides, sulphides, various chemical compounds). Most metals tend to deteriorate in contact with the atmosphere, water or other natural or industrial environments and gradually return to their original compound state. Metal corrosion is equivalent to a chemical dissolution or electrochemical dissolution of a metal or an alloy, causing material losses dependent on the type of material and the nature of the environment (chemical composition, temperature, etc). In the case of steels, the term corrosion is applied to phenomena developed in the liquid phase (aqueous or organic solutions, salts or molten metals), as opposed to oxidation (or "dry" corrosion) which occurs at high temperature.
Corrosion can be limited, even avoided, if the chemical reactions that take place do not lead to the formation of a soluble type of oxide, but rather to the appearance of a stable, protective oxide layer or compound. If these compounds form a thick enough compact layer (called a "passive film" or "passive layer") on the surface of the metal, the risk of corrosion is considerably reduced by the phenomenon of passivation.
Stainless steels are a modern solution to the problem of corrosion. The addition of highly oxidisable elements such as chromium increases the tendency to form stable oxides. Paradoxically, it can be stated that the corrosion resistance of stainless steels is due to the particularly oxidisable nature of one of its elements, chromium, provided that it is present in the proportion of at least 10,5%. Corrosion resistance is obtained by the spontaneous formation in the open air of a very thin passive film, no thicker than about ten nanometers (the equivalent of a protection given by a sheet of paper placed on a 20-storey building). The stability of the passive film is the factor determining the corrosion resistance of stainless steels and it depends on various factors, the main ones being: nature of the corrosive environment, composition of the steel, surface condition, any contact with other materials. When stainless steel is used correctly and the appropriate grade chosen to suit the surrounding medium, the passive film is spontaneously regenerated after accidental damage.
Wrong choices and the prevalence of various conditions, may lead to failure of stainless steels and the emergence of corrosion. In their case, corrosion may not appear with the obvious rust visible in common steels. The effects of corrosion often propagate with fast rate and destructive results. Different media can cause different types of corrosion attack that may vary in nature and appearance, and several forms of corrosion can occur on stainless steels. Below, follows a short presentation of the most common forms of corrosion that can attack stainless steels.
Uniform corrosion occurs when all, or at least a large section, of the passive layer is destroyed. Corrosion reactions then cause a more or less uniform removal of metal from the unprotected surface. Attack by uniform corrosion on stainless steels occurs mainly in acids or in hot alkaline solutions (i.e. sodium and potassium).
Stainless steels generally show good resistance in oxidizing acids such as nitric acid, but are not always able to maintain their passive layer in non-oxidizing acids (like hydrochloric and hydrofluoric acids). Substances, which are neither reducing nor oxidizing, may also affect the corrosivity of acid solutions. Most important are halides, such as chlorides and fluorides, the presence of which increases the corrosivity of both organic and inorganic acids. Even small amounts of halides may affect the corrosion resistance of stainless steels. The resistance to uniform corrosion is generally improved by higher chromium content, since chromium is essential for ensuring the passivity of stainless steels. Nickel is also important as it helps reduce the corrosion rate of depassivated steel. The presence of molybdenum and copper also has a positive effect and enhance the steel’s resistance.
In selecting material, it is important to consider all variations in temperature and chemical composition that might occur in the process environment. 
3. Uniform corrosion on the outside of a steam tube that has been exposed to sulphuric acid.
There are media in which the passive layer might break down locally, while the rest of the layer remains intact. Such media cause so-called localized corrosion. Pitting corrosion is one type of localized corrosion (later we describe more types) and is characterized by attack at small discrete areas. Attack of this kind occurs mainly in neutral or acidic solutions containing chloride.
Chloride ions facilitate a breakdown of the passive layer. A break in the passive film may be considered as a galvanic cell, in which the bare metal becomes the anode while the surrounding area, with an undamaged passive layer, becomes the cathode. This unfavourable anode-to-cathode surface area ratio causes rapid corrosion of the anode.
When the metal corrodes, dissolved metal ions generate an environment with a low pH and chloride ions migrate into the pit to balance the positive charge of the metal ions. Thus the environment inside a growing pit gradually becomes more aggressive and repassivation becomes less likely. As a result, pitting attack often propagates at a high rate, thereby causing corrosion failure in a short time. Since the attack is small at the surface and may be covered by corrosion products, a pit often remains undiscovered until it causes perforation and leakage. 
4. Pitting attack in the heat affected zone inside a but-welded stainless steel pipe.
Crevice corrosion occurs under the same conditions as pitting, i.e. in neutral and acid chloride solutions. However, attack starts more easily in a narrow crevice than on an unshielded surface. The chemical reactions occurring naturally on a stainless surface in an aqueous environment consume oxygen. In the stagnant solution inside a crevice, the supply of new oxidant is restricted. The composition of the solution within the crevice might thus gradually become different from that of the ambient solution. This difference in composition increases the risk for corrosion. Small amounts of dissolved metal ions cause a decrease of the solution pH inside the crevice and the presence of chlorides facilitates an activation of the metal surface. The bare metal surface behaves anodically to the passive areas and the attack propagates according to the same mechanisms as in the case of pitting. Crevice corrosion may be strong even at relatively low temperature.
High chloride concentrations and low pH increase the probability of pitting and crevice corrosion, as do high temperatures and stagnant solutions. The resistance of stainless steels to these types of corrosion increases with increasing contents of chromium and molybdenum. For austenitic and duplex grades, high nitrogen content is also very beneficial. We draw the attention to the fact that a smooth and clean surface generally exhibits the best corrosion resistance. Since crevices are the most vulnerable areas to corrosion, it may be advantageous to use more highly alloyed materials (i.e. 316 instead of 304) in joints (like flanges, bolts etc) than in the pipes of a piping system. The resistance of a construction to crevice corrosion may also be enhanced by overlay welding with a more corrosion resistant material at potential sites of crevice corrosion. Finally, we note that pitting attack initiates more easily in stagnant solutions than in flowing media and that is why equipment should be designed in a way that permits complete drainage.
For the right examination and decision of which type to choose for avoiding the most common form of corrosion - which is pitting - we use the so-called PRE factor. PRE (Pitting Resistance Equivalent) constitutes a measurement of a stainless steel grade's relative resistance to pitting corrosion. The highest is a grade's PRE number, the highest corrosion resistance it enjoys. The formula that gives us the PRE of each grade is the following
PRE = % Cr + 3.3 % Mo + 16 % N
and shows that the resistance to corrosion is the result of the steel's chemical composition and not its atomic structure (austenitic or ferritic). Notable is the absence of nickel in the PRE formula, given that in most cases it does not contribute to the evasion of pitting corrosion. 
5. PRE comparison between popular and austenitic grades.
Stress corrosion cracking is a brittle failure mode caused by the combined effect of tensile stress and a corrosive environment. Like pitting and crevice corrosion, stress corrosion of stainless steels is most frequently caused by solutions containing chloride or very alkaline solutions. However, most cases of stress corrosion cracking on stainless steels occur at high temperature. At elevated temperatures, solutions that are unlikely to cause pitting and crevice corrosion, due to their low concentrations of chlorides and oxidizing chemicals, may give rise to stress corrosion.
Depending on the environment, relatively low engineering loads may provoke stress corrosion. Residual stresses from different manufacturing operations, such as forming and welding, can be high enough to cause failure. In some practical situations annealing at an appropriate temperature can reduce this potential risk, but such an approach is often difficult for large constructions. Coarse grinding induces tensile stresses into the steel surface that may facilitate initiation of stress corrosion.
Most cases of stress corrosion occur at temperatures above 50℃ but failures at ambient temperature have occurred on standard grade austenitic steels, such as 304 and 316 in swimming pool atmospheres.
Stress corrosion attack on stainless steel typically takes the form of thin, branched cracks. Failure caused by stress corrosion cracking often happens abruptly and without warning, due to the high propagation rates of the cracks. A common cause of stress corrosion is the concentration of chlorides from evaporation on hot steel surfaces. The evaporating liquid might be freshwater or other very dilute solutions, which because of their low chloride concentrations are considered as harmless. When the water evaporates, the chloride concentrations of these liquids might, however, become high enough to cause stress corrosion. 
6. Stress corrosion cracking in a stainless steel tube.
Steels with a ferritic or duplex (ferritic-austenitic) structure generally display a very high resistance to chloride-induced stress corrosion attack. Standard austenitic grades, such as 304 and 316, are rather sensitive to this kind of corrosion. High contents of nickel and molybdenum increase the resistance of austenitic grades to stress corrosion and for this reason the grades (EN) 1.4539, 1.4547, 1.4565, 1.4652 show excellent resistance to chloride-induced stress corrosion cracking.
It is well known that a material that is subjected to a cyclic load can fail at loads far below the ultimate tensile stress of the material. If the material is simultaneously exposed to a corrosive environment, failure may take place at even lower loads and after shorter times. This is caused by a form of corrosion known as corrosion fatigue, which has similarities to stress corrosion cracking. Both corrosion forms cause brittle failures. Corrosion fatigue often takes place at ambient temperature and in moderately concentrated solutions that could be considered harmless with regard to other forms of corrosion.
Residual stresses from manufacturing can adversely affect resistance to corrosion fatigue. These may be reduced by an appropriate heat treatment or by the introduction of compressive stresses in the surface, as achieved by shot peening. High mechanical strength increases the resistance of a stainless steel to corrosion fatigue. Duplex steels are thus often superior to conventional austenitic steels. One application where a change from austenitic to duplex steels has reduced the number of failures caused by corrosion fatigue is for suction roll shells in paper machines. 
7. Stress corrosion cracking resistance of mill annealed austenitic and duplex stainless steels in the drop evaporation test with sodium chloride solutions at 120°C (stress that caused cracking shown as a percentage of yield strength).
Intergranular corrosion occurs when austenitic stainless steels are exposed to temperatures in the range 550-850°C where chromium carbides (Fe, Cr)23 C6 can precipitate in the grain boundaries. The chromium content of the carbide precipitates is very high, and since the diffusion rate of chromium in the austenite is low, the alloy adjacent to the grain boundary becomes chromium depleted. Since chromium is essential to passivity, the chromium-depleted region becomes less corrosion resistant than the matrix. In a corrosive environment the depleted area may be depassivated and corrosion will take place in very narrow areas along the grain boundaries. A stainless steel, which has been heat-treated in a way that produces grain boundary precipitates and adjacent chromium depleted zones, is said to be “sensitized”. Sensitization might occur as a result of welding, or of hot forming at an inappropriate temperature.
Measures to increase the resistance of stainless steels to intergranular corrosion caused by chromium carbide precipitation are solution annealing, lowering the carbon content and alloying with titanium or niobium (stabilizing).
Stainless steels are usually delivered from the steel producer in the solution-annealed condition. Solution annealing means heating to temperatures in the range 1000 – 1200°C, at which chromium carbides are dissolved, followed by rapid cooling in air or water. Such an operation leaves the carbon in solid solution in the steel. Steel with a sensitized structure may be recovered by a further solution annealing procedure. A low carbon content (< 0.03%) extends the time required for significant sensitization and is the second measure to decrease the risk of intergranular corrosion. Modern steel making methods enable much lower carbon contents to be achieved in steels than in the past (304L, 316L). 
8. Intergranular corrosion attack.
Stabilized steels, containing titanium or niobium, show good resistance to intergranular corrosion even though their carbon contents may be fairly high. This is due to the fact that titanium and niobium form carbides more easily than chromium. Such carbides precipitate randomly in the grains and no carbon is available to form chromium carbide precipitates in the grain boundaries. Low carbon grades and stabilized grades can be considered as equally resistant to intergranular corrosion, unless exposed for long periods at temperatures above 500°C, in which case stabilized grades are to be preferred.
When two dissimilar materials are connected electrically and immersed in a conductive liquid, an electrolyte, their corrosion performance might differ significantly when compared with the uncoupled metals. As a rule, the less noble material (chemically active), the anode, is more severely attacked, whilst the more noble metal (chemically inert), the cathode, is essentially protected from corrosion. The corrosion attack is normally most evident close to the junction of the two metals. This phenomenon is called galvanic corrosion.
The degree of galvanic effects, as described above, will depend largely on the nature and kinetics of the electrochemical reactions taking place on the surfaces of the two materials forming a galvanic couple. Other factors that influence galvanic corrosion are the difference in the nobility of the two metals, the surface area ratio between the two metals and the conductivity of the solution.
The relative nobility of different conducting materials in a certain environment is indicated by the so-called galvanic series. Such series are valid for one specific environment and are based on measurements of the open circuit potential (OCP) of each material, uncoupled, in that environment. The higher the OCP, the more noble the material. The smaller the difference in OCP between the two metals forming a galvanic couple, the lower the driving force for galvanic attack. It should be noted that changes in electrolyte composition and temperature could cause significant changes in the positioning of different materials in a galvanic series. As long as stainless steels stay passive, they are in most environments nobler than other metallic construction materials and thus form the cathode in most galvanic couples. Galvanic coupling to stainless steels might, on the other hand, increase the corrosion rates of less noble metals such as mild steel, galvanized steel, copper and brass. Galvanic corrosion between different grades of stainless steel is generally not a problem, provided that each grade would be passive if exposed uncoupled in the particular environment. Galvanic corrosion can be prevented by the use of insulated flanges and isolation spools, but such insulators may cause crevice corrosion in chloride solutions.

9. Galvanic series in flowing seawater, 10°C.
A small anode-to-cathode surface area ratio causes an increased corrosion rate of the anode and should thus be avoided. The coating or painting of a less noble material, galvanically coupled to an uncoated stainless steel, should also be avoided, as very high corrosion rates could be obtained on small anodic areas formed where coating defects occur. Coating the more noble metal in a galvanic couple is, on the other hand, an effective way to reduce the risk of galvanic attack.
The conductivity of the electrolyte affects the intensity as well as the location of the attack. A low conductivity tends to reduce the corrosion rate, but the attack can become very concentrated at the area adjacent to the contact site between the two metals.
When stainless steels are exposed to hot gases, chemical reactions take place between the steels, or rather between their alloying elements, and elements or compounds in the gases. As a rule, these reactions will lead to the formation of gas metal interface layers of reaction products. These surface layers are more or less protective against further attack from the gas. In practice, all stainless steels rely on the formation of an oxide layer. All other reaction products either form a porous, less adherent, and hence non-protective scale or are liquid or even volatile and will flux off any existing scale or evaporate. Thus, one condition that must be fulfilled for good high temperature corrosion resistance is that the gas must be oxidizing, i.e. it must contain enough oxygen for the formation of a protective layer, consisting of oxides of one or several of the alloying elements.
There exist several types of high temperature corrosion (oxidation, sulphidation, carburisation and nitridation, furnace gases, etc) depending on the temperature’s level, the time the steel is exposed, the gases’ composition etc. When choosing material for high temperature applications, one must have an extensive knowledge of existing or expected service conditions, such as gas temperature and composition, and material temperature. After consultation with a reputable supplier, one may come to choose the most suitable type of stainless steel for each corresponding high temperature environment.
Atmospheric corrosion is not a unique form of corrosion, but a collective term to denote the corrosion of surfaces in the atmosphere. It may be indoor or outdoor and all corrosion forms can, in principle, be involved. Corrosion of stainless steels is most often induced by halides and mainly chlorides due to their abundance in our environment. When stainless steel is exposed to an aggressive atmosphere it is primarily stained (sometimes referred to as tea staining), but it can also be attacked by localized corrosion with time, particularly at high chloride levels. It should be noted that discoloration of stainless steel is not automatically the result of atmospheric corrosion or even corrosion attack at all; it can be a discoloration from dirt or extraneous rust, not affecting the stainless steel.
Two large application areas can be distinguished where implementation of atmospheric corrosion knowledge is needed: in architectural and structural use. The surface appearance is much more important for architectural use, while superficial corrosion attack can be neglected in structural applications.
Since the corrosion process in the atmosphere is based on wet corrosion it needs a conductive electrolyte for charge transport and dissolution of metal atoms. Therefore, temperature, relative humidity and weather conditions affect the corrosion process on the stainless steel surface, as do polluting gases (i.e. SO2) and other contaminants and aggressive substances that can dissolve into the electrolyte. Moreover, the exposed condition of the stainless steel’s surface is of great importance: sheltering prevents rinsing by rainfall, thus increasing the corrosivity of the environment, and the inclination angle affects the run-off rate. Additional crucial factors are the surface condition and its smoothness. A coarse surface will retain dirt, particles and corrosive chemicals while a smoother surface (BA and 2B) facilitates rinse-off. Finally, one has to take into account that regular cleaning acts decisively for maintaining the metal in ideal condition. 
10. Relative pitting corrosion resistance of welded 4401 (316) after different post weld cleaning treatments.
Below there are three tables that present the suitability of various popular stainless steel grades to different environments.
| Typical indoor environments | Typical outdoor environments | Suitable stainless steel grades |
| Heated | Deserts and artic areas (rural) | 4016, 4521, 4301, 4372, 2101 |
| Non – heated | Dry environment with low pollution | 4016, 4521, 4301, 4372, 2101 |
| Humid environment with low pollution | Coastal environment with low salt concentrations, urban and industrial areas with moderate pollution | 4521, 4301, 4401, 2101, 2304 |
| Polluted urban and industrial environments, coastal areas with moderate salt deposits | 4401, 4438, 4439, 904L, 254 SMO, 4565, 2304, 2205, 2507 | |
| Swimming pool environments, humid industrial areas with (volatile) aggressive chemicals | Heavily polluted industrial environments, marine atmosphere with high salt concentrations in the atmosphere and a combination of marine and industrial environments | 904L, 254 SMO, 4565, 2205, 2507 |
11. Material selection sheet for some ferritic, austenitic and duplex grades in four different environments: rural, urban, industrial and marine with three different corrosion categories, low (L), medium (M) and high (H), respectively.
Type of outdoor environment and corrosion category
Steel grade |
Rural L M H |
Urban L M H |
Industrial L M H |
Marine L M H |
| 1.4003 1.4016 / 430 1.4521 / 444 | o x x + o o - - - | x x x x x x - + o | x x x x x x + o x | x x x x x x + o o |
| 1.4301 / 304 1.4401 / 316 1.4439 / 317 LMN 1.4539 / 904L | + + + - - - - - - - - - | + + o - + + - - - - - - | o o x + + o - - + - - + | + o x + + o - - + - - + |
| 1.4162 / 2101 1.4362 / 2304 1.4462 / 2205 | - - + - - - - - - | - + o - + + - - - | + o x + + o - - + | + o x + + o - - + |
+ = optimum choice, o = may be used with precautions, x = not suitable, - = over-specified 
The generally held view for stainless steels is characterized by some erroneous conceptions that can easily be attributed to widespread ignorance. One misconception is that stainless steel does not have iron, when this is actually its primary constituent (around 70 %). Another incorrect view is the one that considers stainless steel (usually grade 304) as a solution to any kind of corrosion, and thus when a corrosion issue arises, then people accuse the steel that it is not stainless. However, we saw earlier that we have the possibility of choosing among a great variety of stainless steel grades. What one must do is the study and understanding of the conditions prevailing during the material's application and functioning and the consultation with a credible supplier. At the same time, the final user must certainly take a decision for the relation he accepts between the material's cost and its life cycle (there are "everlasting" types that yet cost dearly). Of utmost importance for preserving stainless steel in good condition is its regular cleaning, as well as the procedure of chemical cleaning (pickling/passivation) Chemical cleaning refers to the procedures of pickling and passivation. Pickling is the removal of a thin layer of metal from the surface of the stainless steel. Mixtures of nitric and hydrofluoric acids are usually used for pickling stainless steels. Passivation usually occurs naturally on the surfaces of stainless steels, but it may sometimes be necessary to assist the process with oxidizing acid treatments. Solutions of nitric or citric acid are used for passivating stainless steel surfaces. If the metal's surface is contaminated with grease, oil or inorganic contaminants, then a cleaning operation prior to acid treatment should be carried out. The two operations can be applied to all stainless steel types. with the completion of the construction.
Finally, a widely held misconception is that because ferritic and duplex types are magnetic they are not real stainless steels and will rust like carbon steel. This is wrong. Purely for reasons of atomic structure, some stainless steels are magnetic and some are not. Corrosion resistance is not a matter of atomic structure but one of chemical composition. Magnetism has nothing to do with it. Reference was made earlier to the PRE formula which is used to state each type's resistance to pitting corrosion. Studying the PRE number of both magnetic and non-magnetic types we easily find out that there exist magnetic grades with higher PRE, and thus move resistant, to non-magnetic. Below we present a table with the PRE number of the most popular grades.
Typical chemical composition, % by wt.
| AISI | EN | Cr | Ni | Mo | N | others | PRE |
| ferritic | stainless steels | - | |||||
| 430 | 1.4016 | 16,5 | - | - | - | 17 | |
| 441 | 1.4509 | 18 | - | - | - | Nb, Ti | 18 |
| 444 | 1.4521 | 17,8 | - | 2.1 | 0.01 | Ti | 25 |
| austenitic | stainless steels | ||||||
| 304L | 1.4307 | 18,1 | 8.1 | - | - | - | 18 |
| 316L | 1.4404 | 17,2 | 10.1 | 2.1 | - | - | 24 |
| 904L | 1.4539 | 20 | 25 | 4.3 | - | 1.5 Cu | 34 |
| duplex | stainless steels | ||||||
| S32101 | 1.4162 | 21,5 | 1.5 | 0.3 | 0.22 | 5Mn | 26 |
| S32304 | 1.4362 | 23 | 4.8 | 0.3 | 0.10 | - | 26 |
| S32205 | 1.4462 | 22 | 5.7 | 3.1 | 0.17 | - | 35 |
PRE = % Cr + 3.3 x % Mo + 16 x % N
13. 
14.
Concluding, stainless steel's main properties can be summarized as follows
corrosion resistance
aesthetic appeal
heat resistance
low lifecycle and maintenance cost
full recyclability
biological neutrality (meets EU RoHS requirements)
ease of fabrication and durability
Alloy is the material that is composed from various chemical elements. When it is solid, it is characterized by the participation of all the elements into the crystal structure. That is, the material is crystalline and in this crystal the atoms of its constituents are arranged in space as if they are atoms of the same kind. The crystal structure reflects the geometrical construction of a chemical element. It is called "crystal" in the case of a solid material that exhibits regular geometrical arrangement of its structural constituents. Depending on the geometrical arrangement the atoms or other particles of the element form, the crystal structure is distinguished into seven lattice systems: triclinic, monoclinic, orthorhombic, rhombohedral, tetragonal, hexagonal and cubic.
Annealing see "heat treatments"
Atom see "chemical element"
Carbide is a chemical compound that consists of carbon and a less electronegative element. With the term "electronegative" we describe the chemical property of the tendency an atom has to attract electrons. The highest the value of this property is, the more the element draws electrons towards itself.
Chemical cleaning refers to the procedures of pickling and passivation. Pickling is the removal of a thin layer of metal from the surface of the stainless steel. Mixtures of nitric and hydrofluoric acids are usually used for pickling stainless steels. Passivation usually occurs naturally on the surfaces of stainless steels, but it may sometimes be necessary to assist the process with oxidizing acid treatments. Solutions of nitric or citric acid are used for passivating stainless steel surfaces. If the metal's surface is contaminated with grease, oil or inorganic contaminants, then a cleaning operation prior to acid treatment should be carried out. The two operations can be applied to all stainless steel types.
Chemical element is a chemical substance that cannot change or be divided into simpler forms. The atom is its smallest unit. The atoms consists of Protons - one element's atoms have the same number of protons. They are positively charged and together with the neutrons (that do not have any charge) they constitute the atom's nucleus and are called nucleons Electrons - electronic cloud that surrounds the nucleus with negative charge and smaller mass. The number of the atom's protons that determines the kind of the element is called the atomic number (Z) and constitutes the element's identity number. All the atoms of an element have the same atomic number. The sum of the protons and the neutrons that exist in an atom's nucleus is called the mass number (A).
The atoms are electrically neutral when they have the same number of protons and electrons. The electrons have the capability of moving to neighboring atoms or being shared among them. Atoms that experience deficit or surplus of electrons are named as ions and carry electric charge. When the atoms acquire electrons, then they form ions negatively charged and are called anions. On the contrary, when they lose electrons they turn into cations, with positive electric charge. When the atoms form chemical compounds they seek to acquire the least possible energy. This takes place with the following chemical bonds. COVALENT BOND is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. NON COVALENT BOND is the exchange of electrons with other atoms.
The electrons' number at the outermost electron shell (cloud) of the atom governs its chemical bonding behavior. These are called valence electrons and are those that can participate in the formation of chemical bonds with other atoms. Valence electrons can determine the element's chemical properties and if it will bond with others or not. Valence electrons are important in determining how the atom reacts chemically with other atoms. Atoms with a complete (closed) shell of valence electrons tend to be chemically inert (i.e. noble gases as opposed to alkali metals that have only one electron - for example sodium and potassium). Atoms with one or two valence electrons more than a closed shell are highly reactive because the extra electrons are easily removed to form positive ions. Atoms with one or two valence electrons fewer than a closed shell (i.e. halogens - for example fluorine and chlorine) are also highly reactive because of a tendency either to gain the missing electrons and form negative ions, or to share electrons and form covalent bonds. The number of valence electrons of an element is determined by its periodic table group in which the element is categorized.
The periodic table is a tabular display of the chemical elements, organized on the basis of their properties. It accurately predicts the properties of various elements and the relations between properties. As a result, it provides a useful framework for analyzing chemical behavior, and is widely used in chemistry and other sciences. Elements are presented in increasing atomic number. The table classifies the elements according to their similarities and differences. In this table, the elements are presented in vertical columns (groups) and horizontal rows (periods) in consecutive number of their atomic number. The position and the properties of each element are determined by the number of the valence electrons at the outermost shell of their atoms. Therefore, the elements found in the same column of the periodic table have the same number of valence electrons and thus similar chemical properties. While the elements found in the same row (period) allocate their electrons on the same shell number (in their basic condition), presenting as a result similar physical properties. As chemical properties we refer to the characteristics of an element that influence its behavior when it reacts with other elements under any conditions and are dependent on its chemical composition. Physical properties are the general features of a material in its natural form. That is, the state in which the material exists (solid, liquid, gas), its temperature, size etc. The physical properties are not dependent on the element's chemical composition.
We note that the main alloying elements that are present in stainless steels are near to each other in the periodic table, having similar atomic number (Ti 22, Cr 24, Mn 25, Fe 26, Ni 28).
Heat treatments are a group of industrial and metalworking processes used to alter intentionally the steel's microstructure with the aim of shaping its properties. Heat treatment involves the use of heating or chilling, normally to extreme temperatures, to achieve a desired result such as hardening or softening of a material. Annealing is the most important heat-treating process. It consists of heating a metal to a specific temperature and then cooling at a rate what will produce a refined microstructure. Annealing is most often used to soften a metal for cold working, to improve machinability, or to enhance properties like electrical conductivity. Annealing includes the heating of the material in specific temperature and for specific time interval, and then cooling slowly in room temperature. It is used to remove the hardness caused by cold working and to repair the defects caused by plastic deformation. Annealing is distinguished to:
Full annealing which aims to soften the steel before any machine working. The cooling takes place at very slow rates, usually inside an oven.
Normalizing aims at the homogeneity of the alloying elements for achieving the best transformation of the austenite during cooling. It includes heating in the austenite region with temperatures higher than those applied in full annealing. The metal is then cooled in open air. It is a technique used to provide uniformity in grain size and composition throughout an alloy. Normalizing gives harder and stronger steel, but with less ductility for the same composition, than full annealing.
Stress relief annealing is a technique to remove or reduce the internal stresses created in a metal. These stresses may be caused in a number of ways, ranging from cold working to non-uniform cooling. Stress relieving is usually accomplished by heating a metal below the lower critical temperature and then cooling uniformly.
Heat treatments are also used for the strengthening of steel. A classical heat-treating method for this purpose is quenching and tempering. After the steel's austenization in high temperature, where the carbides of the alloying elements are dissolved, the steel is subjected to quenching by a medium (air or other gases, oil or water) depending on the alloy and other considerations (such as concern for maximum hardness vs. cracking and distortion). Therefore, by being rapidly cooled, a portion of austenite (dependent on alloy composition) will transform to martensite, a hard, brittle crystalline structure. The quenched hardness of a metal depends on its composition and quenching method. Cooling speeds, from fastest to slowest, go from fresh water, oil and forced air. Quenching is followed by tempering as untempered martensitic steel, while very hard, is too brittle to be used for most applications. Tempering is the method used for alleviating this problem and it consists of heating a steel below the lower critical temperature (often from 205 to 595℃ depending on the desired results), to impart some toughness.
Finally, we note the presence of surface treatments that affect only the surface of an engineering material (i.e. carburisation and nitridation which describe the enrichment with carbon and nitrogen for higher surface hardness).
Ion see "chemical element"
Iron the chemical element symbolized by Fe is a metal with atomic number 26, melting and boiling temperatures 1535℃ and 2750℃ respectively. It is the forth most abundant element on the earth's crust after oxygen (O), silicon (Si), and aluminum (Al). Moreover, it is the metal with the widest use, especially in the form of its two most important alloys, that of steel and that of cast-iron (iron alloy with carbon C > 2,1 %).
Mechanical properties of a metallic alloy are those that describe the material's ability to compress, stretch, bend, scratch, dent or break. The most commonly used criteria for evaluating mechanical characteristics are: a. Strength: the degree of resistance of a material to deformation. Two critical values are generally considered: yield strength, or the stress the material can be subjected to before permanent plastic deformation occurs tensile strength, or the stress it can be subjected to before rupture/failure 
15. UTS (ultimate tensile strength) is measured in MPa (1Mpa=1N/mm3=145PSI=0.1 kg/mm3) and represents maximum resistance at failure. YS (yield strength) refers to the beginning of the "plastic" phase, where elongation no longer disappears when the stress is removed.
b. Hardness: the degree of resistance to indentation by an applied load. c. Toughness: the capacity to absorb deformation energy before fracture. d. Ductility (or plasticity): the ability to deform plastically without fracturing. 
16. Rm=ultimate tensile strength, Rp02=yield strength and A5/A80=elongation to fracture.
Metal is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny. Most of them have high density and are hard and tough (iron, copper, aluminum, sodium, calcium, zinc, magnesium, titanium, uranium, etc). Except from mercury, they are all solids in room temperature 20℃. In a metal, atoms readily lose electrons to form positive ions (cations). Those ions are surrounded by de-localized electrons, which are responsible for the electrical conductivity. The solid thus produced is held by electrostatic interactions between the ions and the electron cloud, which are called metallic bonds. The metals are extracted from minerals through a series of procedures described by the term of metallurgy. Apart from metals, there exist two more important categories of chemical elements: the non-metallic and the metalloids.
Microstructure is defined as the structure of a prepared surface or thin foil of material as revealed by a microscope above 25 X magnification. The microstructure of a material (which can be broadly classified into metallic, polymeric, ceramic and composite) can strongly influence its properties such as strength, toughness, ductility, hardness, corrosion resistance, high/low temperature behavior, wear resistance, and so on, which in turn govern the application of these materials in industrial practice. 
17. Increasing the nickel content changes the microstructure of the stainless steels.
Mineral is a naturally occurring solid chemical substance formed through biogeochemical processes, having characteristic chemical composition, highly ordered atomic structure (usually crystalline), and specific physical properties. By comparison, a rock is an aggregate of mineral and/or mineraloids and does not have a specific chemical composition.
pH is a way of expressing the concentration of hydrogen's ions into a liquid solution. With pH we symbolize the negative decimal logarithm of the hydrogen ions' [H+] concentration in the solution pH = -log[H+]. The pH level is a unit of measure describing the degree of acidity or alkalinity of a solution. This is measured on a scale of 0 to 14.
pH < 7 - acid solutions (i.e. coffee, vinegar, lemon juice, gastric fluids)
pH > 7 - alkaline solutions (i.e. saliva, blood, seawater, chlorine)
pH = 7 - neutral solutions (pure water)
Phase in the physical sciences is a region of space (a thermodynamic system), through out which all physical properties of a material are essentially uniform. A simple description is that a phase is a region of material that is chemically uniform, physically distinct and often mechanically separable. A material's transition from one phase to another has to do with changes in its state (i.e. from solid to liquid) or more imperceptible changes like those related to its crystal structure.
Physical properties of a metallic alloy concern the material's ability to conduct heat, conduct electricity, expand or shrink. 
18. Physical properties
Solid solution is a solid-state solution of one or more solutes in a solvent. Such a mixture is considered a solution rather than a compound when the crystal structure of the solvent remains unchanged by addition of the solutes, and when the mixture remains in a single homogeneous phase. This often happens when the two elements (generally metals) involved are close together on the periodic table. Conversely, a chemical compound is generally a result of the non-proximity of the two metals involved on the periodic table. Stabilizer is an element that prevents carbide formation, primarily along the grain boundaries. To this purpose, stainless steel is alloyed with titanium and niobium. These stabilizing elements preferentially combine with the excess carbon in the steel, thereby leaving the chromium to perform its function of forming and maintaining a stable oxide layer (passive film). Tensile strength see "mechanical properties" Yield strength see "mechanical properties"
With each customer's needs in mind, we need your feedback to continuously improve our services!